Methotrexate (MTX) is a dihydrofolate reductase-inhibiting antimetabolite widely used to treat moderate–severe psoriasis.1 Owing to its mechanism of action, it can precipitate acute gout flares; although this adverse effect was described long ago,2 it is likely underreported given the high prevalence of gout in the general population (2.4% in Spain).3
A 70-year-old man with untreated gout and hyperuricemia and a 30-year history of plaque psoriasis, managed with topical corticosteroids and without a history of psoriatic arthritis, presented with a severe psoriasis flare (PASI 20, BSA 47%). Subcutaneous MTX 15mg weekly was started. Two months later he presented to the emergency department with arthritis of the right first metatarsophalangeal joint. Given the gout history, a normal radiograph, and a serum urate of 8.1mg/dL (normal≤6.8mg/dL), the episode was diagnosed as podagra. At dermatology follow-up, MTX was stopped and phototherapy (UVB) initiated. Because psoriasis improved only modestly, subcutaneous MTX was reintroduced at 12.5mg weekly after 3 months; 15 days later he experienced another gout attack. As this was the second episode after starting MTX, it was discontinued permanently. The patient's psoriasis is now well controlled on acitretin 25mg three times per week, with no new gout attacks after 1 year of follow-up.
Gout is a chronic inflammatory arthropathy due to intra-articular deposition of monosodium urate crystals in certain hyperuricemic patients.4 It presents with recurrent arthritis flares, most often involving the first metatarsophalangeal joint.4 Gout attacks can be precipitated by many factors (drugs, infections, alcohol, fasting, specific foods, physical trauma, etc.) as serum urate levels change—either increase or decrease—and via a synergistic proinflammatory effect of monosodium urate crystals with saturated fatty acids such as stearic acid, activating Toll-like receptor 2 and inducing interleukin-1β release.5
Patients with psoriasis have an increased risk of hyperuricemia and gout.6,7 This is partly attributable to the chronic systemic inflammation underlying psoriasis, with involvement of inflammatory pathways shared with gouty arthritis, notably type 1 and type 17 (Th1/Th17) lymphocyte-mediated pathways.7 In addition, the high keratinocyte turnover in psoriasis increases urate production from purine metabolism, leading to secondary hyperuricemia.2,7,8
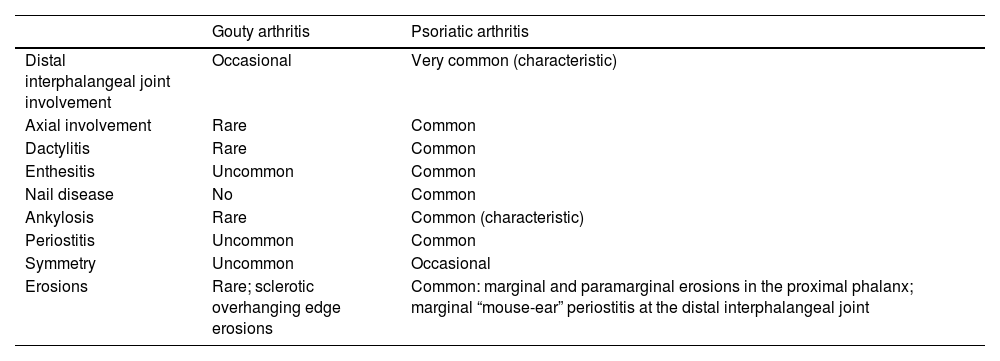
The risk of gout appears even higher in patients with psoriatic arthritis, especially in middle-aged adults (31–50 years).7 Monosodium urate crystals are more frequently found in synovial fluid from patients with psoriatic arthritis.9 Serum urate levels also correlate with psoriasis severity, and psoriasis treatment seems to reduce them.7,10 Consequently, arthritis in patients with psoriasis and hyperuricemia may raise a differential diagnosis between gouty arthritis and psoriatic arthritis, whose main distinguishing features are summarized in Table 1.
Differential clinical and radiologic findings between psoriatic arthritis and gouty arthritis.
| Gouty arthritis | Psoriatic arthritis | |
|---|---|---|
| Distal interphalangeal joint involvement | Occasional | Very common (characteristic) |
| Axial involvement | Rare | Common |
| Dactylitis | Rare | Common |
| Enthesitis | Uncommon | Common |
| Nail disease | No | Common |
| Ankylosis | Rare | Common (characteristic) |
| Periostitis | Uncommon | Common |
| Symmetry | Uncommon | Occasional |
| Erosions | Rare; sclerotic overhanging edge erosions | Common: marginal and paramarginal erosions in the proximal phalanx; marginal “mouse-ear” periostitis at the distal interphalangeal joint |
Patients with gout have a higher risk of metabolic syndrome and cardiovascular disease.7 The high prevalence of hyperuricemia and gout among patients with psoriasis may contribute to the well-known metabolic and cardiovascular comorbidities in psoriasis, although a causal relationship has not been established.7
MTX has been used safely in patients with gout. In a recent clinical trial,11 the safety and efficacy profile of MTX in patients with refractory gout treated with pegloticase were evaluated. The MTX–pegloticase group achieved a higher urate response rate than the pegloticase-only group, without significant differences in the number of gout flares. However, MTX can alter serum urate via several mechanisms: inhibition of purine synthesis through dihydrofolate reductase; anti-inflammatory effects mediated by adenosine release and inhibition of T-cell proliferation; and modification of renal excretion.2,8,12 However, MTX-induced changes in urate levels may trigger gout episodes in predisposed patients, as in our case.
Some authors have suggested prophylactic colchicine before initiating MTX,2 although clinical guidelines provide no specific recommendations. In our view, preventive therapy is not necessary, but a history of gout should be considered to anticipate possible attacks or to enable early intervention should one occur.
In conclusion, we report a patient with MTX-triggered gouty arthritis, to our knowledge the second such case described in the literature. Dermatologists should be aware of this potential adverse effect and suspect it when a plausible temporal relationship exists between a gout flare and MTX initiation. Although MTX was withdrawn in our case per patient preference, the preferred approach is to treat the gout—i.e., anti-inflammatory therapy for the flare and, especially, urate-lowering therapy—and MTX discontinuation is not necessarily required to prevent further gouty arthritis episodes.
Conflicts of interestNone declared.