Actinic keratosis (AK) is a highly prevalent premalignant lesion. The risk of progression to squamous cell carcinoma varies between 0% and 0.53% per AK lesion per year.1 A wide variety of therapeutic options are available. Cryotherapy and curettage are among the options available for isolated lesions. However, it is relatively common for patients to present field cancerization, requiring treatment of the entire area, including 5-fluorouracil (5-FU), imiquimod, ingenol mebutate, or photodynamic therapy (PDT).1,2 Evidence regarding which is the most effective treatment is scarce.
Recently, Jansen et al3 published the results of the first multicenter randomized, blind clinical trial (n = 624), performed in the Netherlands, which compared 5-FU 5% (n = 155) (applied twice daily for 4 weeks), imiquimod 5% (n = 156), ingenol mebutate 0.015% (n = 157), and PDT with methyl aminolevulinate (PDT-MAL) (n = 156). Imiquimod, ingenol mebutate, and PDT-MAL were used in accordance with the product information sheet. The study included adults with 5 AK lesions or more (of any grade) in an area of between 25 and 100 cm2 on the head or neck. Immunocompromised individuals were excluded from the study. In the first visit, a transparent sheet was drawn over the exact position of the AK lesions to allow for an accurate follow-up. Patients were able to receive up to 2 treatment cycles. The main objective was to evaluate a reduction of 75% or more in the AK lesions after 12 months. Therapeutic failure was defined as failure to achieve this percentage.
An intention-to-treat analysis was performed on the 602 patients with available clinical-response data. The probability of not presenting treatment failure was 74.7% (confidence interval [CI], 66.8–81) with 5-FU, 53.9% (CI, 66.8–81) with imiquimod, 37.7% (CI, 30–45.3) with PDT-MAL, and 28.9% (CI, 21.8–36.3) with ingenol mebutate. The differences between 5-FU and the other treatments were statistically significant. The per-protocol analysis showed similar results. The relative risk of therapeutic failure when compared with 5-FU (hazard ratio = 1) was 2.03 with imiquimod, 2.73 with PDT-MAL, and 3.33 with ingenol mebutate. Satisfaction with treatment and improved quality of life was higher with 5-FU than with the other drugs (Table 1). Treatment failure after the first treatment cycle was observed in 14.8% with 5-FU, in 37.2% with imiquimod, 34.6% with PDT-MAL, and 47.8% with ingenol mebutate.
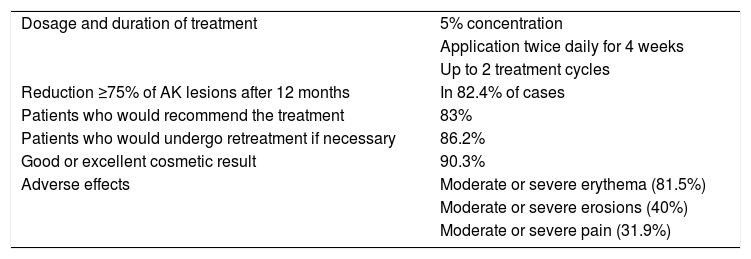
Characteristics, Treatment Response, and Adverse Effects of Treatment With 5-Fluorouracil in the Clinical Trial by Jansen et al.3.
| Dosage and duration of treatment | 5% concentration |
| Application twice daily for 4 weeks | |
| Up to 2 treatment cycles | |
| Reduction ≥75% of AK lesions after 12 months | In 82.4% of cases |
| Patients who would recommend the treatment | 83% |
| Patients who would undergo retreatment if necessary | 86.2% |
| Good or excellent cosmetic result | 90.3% |
| Adverse effects | Moderate or severe erythema (81.5%) |
| Moderate or severe erosions (40%) | |
| Moderate or severe pain (31.9%) |
Abbreviation: AK indicates actinic keratosis.
Adherence to treatment exceeded 88% with all treatments. The rate of adverse effects was similar in all groups. However, PDT-MAL was associated with more pain. No associated severe adverse effects were observed and none of the patients suspended the drug for this reason.
This study shows that 5-FU may be superior and have no more adverse effects than other therapeutic alternatives, and patients present a high degree of satisfaction. One of the disadvantages in Spain is the lack of a commercial presentation of 5% 5-FU, which makes it necessary to formulate the drug and may limit its prescription.
5-FU is an effective, safe, and economical alternative in the management of AK. This new evidence demonstrates its superiority to imiquimod, PDT-MAL, and ingenol mebutate.
Please cite this article as: Morgado-Carrasco D, Fustà-Novell X, Toll A. FR-Nueva evidencia a favor de 5-fluorouracilo en eltratamiento de lasqueratosis actínicas. Actas Dermosifiliogr. 2021;112:69–70.