Juvenile dermatomyositis (JDM) is an autoimmune disease characterized by small vessel inflammation. It is uncommon in infants and shows involvement of the skin, muscles, and other organs. The mean age at diagnosis is 7 years. The estimated incidence is 1.9–4 cases per million inhabitants/year, with a predominance in girls of 2.3:1.1,2
During the SARS-CoV-2 pandemic, an increase in the incidence of cases of JDM was observed in our hospital in comparison with the preceding 20 years. Between 1999 and 2019, 8 cases of DMJ were diagnosed, whereas from March 2020 through November 2021, 5 new cases were reported. All patients presented clinical findings characteristic of JDM, but we also observed involvement of the oral mucosa, ears, hands, and feet; these are uncommon findings in the literature.
The 5 patients with JDM diagnosed during the pandemic comprised 3 boys and 2 girls, aged between 8 and 13 years. None of these patients had a history of infectious disease or vaccination prior to the onset of clinical symptoms. In all cases, the most frequent symptom was asthenia, which presented before the skin manifestations. Proximal muscle weakness and findings typical of dermatomyositis, such as heliotrope rash, malar rash, periungual erythema and dilated capillaries, cuticular abnormalities, and Gottron papules and erythematosquamous plaques on the elbows and knees were present in all cases. Telangiectasias of the eyelids were observed in 2 patients. We did not find any inverse Gottron papules, flagellate erythema, or shawl-sign erythema.
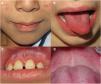
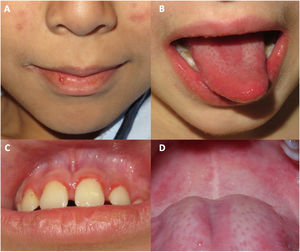
Oral mucosal involvement was present in all cases, presenting as cheilitis, gingivitis, and depapillation or geographic tongue (Fig. 1).
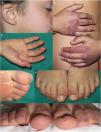
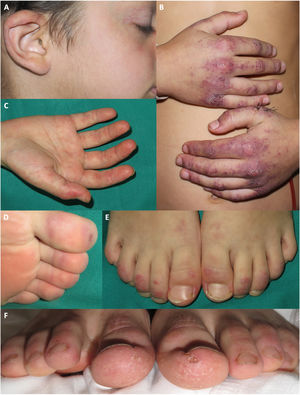
Three patients presented erythema or erythematosquamous papules on the ears. Three patients had palmar erythema. Four patients presented chilblain-like lesions on the feet. Two boys had scaling on the fingers and toes (Fig. 2). Four of the five patients reported itching associated with the skin lesions.
Acral manifestations in patients diagnosed with juvenile dermatomyositis in our hospital during the COVID pandemic. A, Erythematous and purpuric plaques on the ear of a patient who also presented telangiectasias on the eyelids and cheilitis. B, Purpuric erythematous plaques with scaling on the backs of the hands, with more extensive involvement than that observed with typical Gottron papules. C, Palmar erythema. D, Purpuric macules on the toes. E, Erythematous-desquamative plaques on the backs of the toes. F, Erythematous-desquamative plaques on the toes, associated with scaling, fissuring, and ulcer in the plantar region of the toes.
The most common laboratory findings were elevated creatinine kinase, aldolase, lactate dehydrogenase, and glutamic oxaloacetic transaminase or glutamic pyruvic transaminase. In all cases, the polymerase chain reaction test for SARS-CoV-2 was negative at the time of diagnosis. Specific antibodies for dermatomyositis were studied, with anti-TIF1-gamma (P155) positive in 2 patients and anti-MDA-5 (CADM140) positive in another 2; one of the girls was negative for these specific antibodies (Table 1).
Clinical and laboratory findings in patients diagnosed with juvenile dermatomyositis in our hospital during the COVID pandemic.
| Patient | Sex | Age at diagnosis, y | Duration of lesions prior to the consultation, m | Fever | Arthralgia | Myalgia | Arthritis | Telangiectasias on the eyelids | Ear lesions | Cheilitis | Gingival involvement | Depapillation or geographic tongue | Palmar erythema | Scaling of the fingers | Chilblain-like lesions on the feet | Diffuse alopecia | Pruritus | DM specific antibodies |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 13 | 6 | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | No | No | Yes | Yes | No | Negative |
| 2 | M | 8 | 1 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | TIF1-gamma |
| 3 | F | 10 | 6 | No | No | Yes | No | No | Yes | Yes | Yes | Yes | No | No | No | No | Yes | TIF1-gamma |
| 4 | M | 13 | 1 | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | Yes | No | Yes | MDAS |
| 5 | M | 9 | 3 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | MDAS |
Abbreviations: DM, dermatomyositis; F, female; M, male.
In all cases, nuclear magnetic resonance imaging (MRI) revealed muscular edema, a finding compatible with JDM, and so it was not necessary to perform muscular biopsy to confirm diagnosis.
After several months of follow-up, all patients had progressed favorably in terms of symptoms, cutaneous-mucosal signs, and laboratory parameters after initiating treatment with topical corticosteroids and calcineurin inhibitors, systemic corticosteroids, methotrexate, hydroxychloroquine, or immunoglobulins.
JDM is a connective tissue disorder characterized by skin involvement, characteristically associated with proximal muscle weakness.2
Recently, a case series has been published of patients with JDM associated with COVID.3 Epitopes identical to SARS-CoV-2 proteins have been identified in patients with dermatomyositis.4 In addition, it has been proposed that SARS-CoV-2 infection could trigger the development of JDM, probably by stimulating the IFNα pathway.
Our patients did not show any signs or symptoms of COVID prior to onset of JDM, and a PCR test for SARS-CoV-2 at the time of diagnosis of JDM was negative. The presence of chilblain-like lesions in 4 patients could correspond to COVID fingers, and be a late manifestation of asymptomatic or oligosymptomatic infection due to COVID.5 In addition, 2 patients presented with scaling on both fingers and one of them experienced ulcers, similar to those known as hikers foot.6
Oral manifestations in patients with JDM have not been widely reported in the literature. Cases have been described of gingival vasculopathy and telangiectasias, cheilitis, erythematous plaques in the palate, and depapillation or geographic tongue. All these findings were present in our patients.7,8
As in other published series, in our patients, the skin lesions and photosensitivity persisted for months, despite starting effective systemic treatment.9
Recently, antibodies specific to dermatomyositis have been reported associated with certain phenotypes and prognoses.2 MRI can confirm muscle involvement, thereby reducing the need for invasive tests such as muscle biopsy or electromyograph.10
Intensive treatment from diagnosis of JDM is essential for improved prognosis. A larger case series would be needed to establish whether there is a relationship in patients with JDM between involvement of the oral cavity and acral areas, both with SARS-CoV-2 infection and long-term prognosis.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Dr. Jan Ramakers of the Infant Rheumatology Department and Dr. Juan Garcías-Ladaria of the Dermatology Department of the Hospital Universitari Son Espases, Spain, for their collaboration in the diagnosis and treatment of patients and their comments on the manuscript.