Lentigo maligna is the most common type of facial melanoma. Diagnosis is complicated, however, as it shares clinical and dermoscopic characteristics with other cutaneous lesions of the face. Reflectance confocal microscopy is an imaging technique that permits the visualization of characteristic features of lentigo maligna. These include a disrupted honeycomb pattern and pagetoid cells with a tendency to show folliculotropism. These cells typically have a dendritic morphology, although they may also appear as round cells measuring over 20μm with atypical nuclei. Poorly defined dermal papillae and atypical cells may be seen at the dermal-epidermal junction and can form bridges resembling mitochondrial structures. Other characteristic findings include junctional swelling with atypical cells located around the follicles, resembling caput medusae. Reflectance confocal microscopy is a very useful tool for diagnosing lentigo maligna.
El lentigo maligno es el melanoma más frecuente en la cara.
El diagnóstico del lentigo maligno es complicado porque los signos clínicos y dermatoscópicos asociados a lentigo maligno pueden verse en otras lesiones cutáneas faciales.
La microscopia confocal de reflectancia es una técnica de imagen que permite detectar hallazgos característicos del lentigo maligno. En la epidermis encontramos la pérdida del patrón en panal de abejas y células pagetoides con tendencia al foliculotropismo. Estas células pagetoides suelen ser de morfología dendrítica, aunque también pueden presentarse como células redondas mayores de 20μm con núcleos atípicos. En la unión dermoepidérmica las papilas dérmicas pueden estar mal delimitadas y haber células atípicas. Estas células pueden formar puentes que parecen estructuras mitocondriales. Además, podemos ver engrosamientos junturales con células atípicas localizados alrededor de los folículos simulando una cabeza de medusa.
La microscopia confocal de reflectancia es muy útil en el diagnóstico del lentigo maligno.
Lentigo maligna (LM) is a melanoma in situ that usually presents on sun-exposed skin (head and neck) in elderly individuals. LM accounts for 80% of melanomas in situ and is the most common facial melanoma. If the lesion becomes invasive, it is termed LM melanoma (LMM).1
Diagnosis of LM is difficult because the initial clinical and dermoscopic signs are subtle and not pathognomonic. In addition, differential diagnosis needs to include numerous pigmented lesions, such as solar lentigines (SL), simple lentigines, flat seborrheic keratosis (irritated or not irritated), lichenoid keratosis, actinic keratosis (above all, the pigmented variety), Bowen disease, and pigmented basal cell epitheliomas.2,3
Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that permits visualization of the epidermis and papillary dermis with a cellular resolution approaching that of conventional histology. RCM is an excellent technique for analyzing and establishing diagnosis of facial lesions. This article describes the main features of confocal microscopy images associated with diagnosis of LM.
Clinical, Histological, and Dermoscopic Features of Lentigo MalignaLM is characterized clinically by the presence of slow-growing pigmented macules. Differential diagnosis with solar lentigines, pigmented actinic keratosis, and flat seborrheic keratosis is sometimes complex. It is much easier to distinguish LM from melanocytic nevi on the face given that, in elderly individuals, these latter lesions often have a papular appearance, with normal skin coloration (Miescher nevus).
Histologically, differential diagnosis between LM lesions and atypical melanocytic hyperplasia associated with sun-damaged skin may also be difficult, particularly when only small biopsy samples are available. A good pathologic-clinical correlation is essential for avoid wrong diagnosis, particularly in areas of sun-damaged skin such as the face and neck in elderly individuals and in large lesions.4 If LM is suspected on clinical grounds and the pathologic diagnosis is of junctional nevus, dysplastic nevus, or atypical lentiginous nevus, the pathology findings should be reviewed in detail. Furthermore, surgery is usually complicated as these are large lesions with poorly defined borders on the face. With amelanotic forms, recurrence is not uncommon.
Dermoscopy is a technique that increases the sensitivity and specificity of diagnosis of pigmented facial lesions. The classic dermoscopic features associated with LM were described by Schiffner et al.5 in 2002 and include asymmetric follicular pigmentation, presence of small slate-gray dots and globules irregularly distributed around the follicle (annular-granular pattern), pigmented rhomboidal structures, and homogeneous areas that obliterate the follicles. Actinic pigmented keratosis, SL, and lichenoid keratosis can also present an annular-granular pattern (grey dots/globules). The rhomboidal structures and, above all, the homogeneous areas that obliterate the follicles are more specific to LM and are mainly associated with LM that have become invasive.6
In 2012, Pralong et al.7 published the characteristics of 125 LM/LMM lesions in white patients, and verified the utility of the classic signs of LM. They also identified additional dermoscopic signs associated with LM. These included increased vascular density in the region of LM compared to surrounding skin, presence of red rhomboidal structures, presence of bullseye structures, and darkening of the lesion on examination with dermoscopy compared to the clinical examination.
The increased vascular density and red rhomboidal structures can also be seen in actinic keratosis.
The angular or zigzag lines described in LM correspond to the early stages of formation of rhomboidal structures.8 In some extrafacial LM, the only dermoscopic sign may be these zigzag lines.9 The presence of fine dots similar to those seen in the annular-granular pattern, but of brown color and with nonannular distribution, has been associated with failure of radiotherapy or imiquimod treatment.10
Confocal Reflectance Microscopy Features of Lentigo MalignaRCM is a noninvasive imaging technique that permits visualization of the epidermis and papillary dermis with a cellular resolution approaching that of conventional histology. Both clinical and dermoscopic differential diagnosis of macular facial lesions such as SL, seborrheic keratosis, actinic keratosis, Bowen disease, basal cell epithelioma, and LM is sometimes difficult. In these cases, RCM is very useful for establishing the correct diagnosis. Most of the changes in LM are located in the epidermis and the dermal-epidermal junction. RCM permits a precise visualization of the architectural and cytologic features typical of LM because images are obtained from the epidermis to the papillary dermis.11 In addition to diagnosis, RCM can also be used to define the margins of LM prior to treatment12–14 as well as during surgery.15 Furthermore, RCM can detect recurrence after surgery or be used to assess response to nonsurgical treatments.10,12,16,17
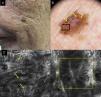
A focal or global loss of honeycomb architecture is detected by RCM in the epidermis of LM lesions. Atypical cells corresponding to pagetoid cell infiltration in histology are also observed. The cells present are mainly of dendritic morphology (large and pleomorphic cells with prominent dendrites) and very indicative of LM if they are more than twice the size of keratinocytes, abundant and atypical, and located around follicles (Fig. 1). Pagetoid cells can also be round, large, and atypical, with evident nuclei. Round cells occur less frequently than dendritic cells in LM but if they measure more than 20μm they are more specific.
A, Facial polychrome macule of 3 years duration on a 68-year-old man. B, Dermoscopic image with asymmetric follicular openings (yellow arrow), increased vascular density (yellow square), and brown rhomboid structures (blue arrow). C, RCM image corresponding to the area of the 1×1.5mm black square in the dermoscopic image, showing abundant dendritic cells (yellow square) with follicular localization. To the left of the image, loss of the honeycomb pattern is observed in the spinous cell layer; to the right, the image corresponds to the horny layer.
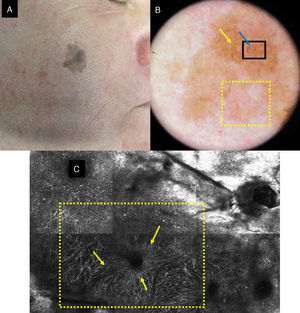
A loss of the annular pattern at the dermal-epidermal junction is a common finding. The presence of poorly delineated papillae with atypical cells is very indicative of LM. It is necessary to differentiate between failure to visualize papillae characteristic of the facial site and the presence of papillae with poorly defined edges. Dendritic cells at the dermal-epidermal junction can form bridges that resemble mitochondria. These structures are frequently observed in melanomas in situ (Fig. 2).18 Junctional thickening with atypical cells can also be found in LM. This junctional thickening can be located radially around follicles, giving them a tentacle-like appearance (Fig. 2d).19,20
A, Clinical image showing a pigmented lesion on an 80-year-old man. B, Dermoscopic lesion with abundant blue-gray rhomboid structures (yellow arrows) and vascularization (blue arrow). C, CRM image corresponding to the area of the 1×1mm black box in the dermoscopic image, showing the dermal-epidermal junction broadening with atypical cells (yellow arrows); the localization of the dendritic cells, forming bridges that constitute the structures that simulate mitochondria (blue arrows) can also be seen. D, CRM image of 1×1mm in which their follicular localization can be seen with a tentacle-like appearance (yellow box).
Atypical nucleated cells can occasionally be observed in the dermis.
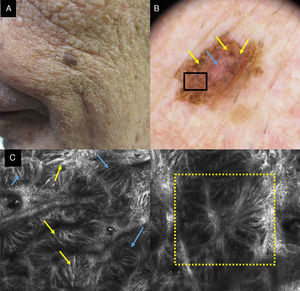
With RCM, we can distinguish between LM and SL, which is the main entity to be included in the differential diagnosis (Fig. 3). In SL, the main findings are located in the dermal-epidermal junction, as the changes are observed in the papillary dermis and rete ridges. There is an increase in the density of the dermal papillae that presents as bright contours in a polycyclic shape (papillae with polycyclic outline). These contours are also known as bulbous projections and are formed by monomorphic, brilliant, reflective cells without atypia, which contain melanin and melanosomes. The contours seen by RCM correspond in dermoscopy to the fingerprint. In the granular and spinous layer, SL lesions maintain the honeycomb pattern consisting of monomorphic polygonal cells with a dark nucleus and clear cytoplasm, and melanophages and lymphocytes can be seen in the dermal papillae. The honeycomb pattern is lost focally or globally in LM lesions. LM lesions also often show atypical pagetoid cells with a dendritic appearance with folliculotropism as well as loss of papillae and nonhomogeneous junction thickening with atypical cells.21
A, Dermoscopic image of a lesion of 3 years duration on the cheek of a 72-year-old woman. Dark brown rhomboidal structures are observed on a large part of the lesion (yellow arrows). B, CRM image showing irregular junction broadening with atypical cells (yellow arrows). C, Dermoscopic image of a supraciliary lesion of 10 years duration in a 50-year-old woman. Asymmetric follicular pigmentation (yellow arrows) can be observed. D, CRM image showing areas (yellow boxes) with an increased density of dermal papillae with polycyclic and geometric outlines that are named polycyclic papillary contours (also known as bulbous projections).
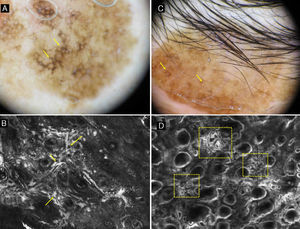
RCM also permits diagnosis of lichenoid keratosis. Clinically, this condition presents as macular lesions which, in dermoscopic images, show blue-grey regression distributed throughout the lesion. With RCM, pagetoid cells and cellular atypia are not observed. These are abundant plump bright cells that sometimes cluster together and that correspond to melanophages, usually without a visible nucleus.22
In 2010, Guitera et al.19 published a prospective study analyzing 64 signs observed in RCM in hard-to-diagnose facial lesions (81 LM lesions and 203 benign lesions were included). They described the confocal features of the LM lesions, and identified 6 parameters that were related to diagnosis of LM and established a diagnostic algorithm. This included 2 major criteria, each of which score +2 points, and 4 minor criteria, 3 of which scored +1 point and 1, a negative criterion that protected diagnosis of LM, scored -1. The major criteria included loss of reflectance annular pattern typical of the dermal-epidermal junction and the presence of round pagetoid cells measuring more than 20 microns across. The positive minor criteria were presence of 3 or more atypical cells in 5 quadrants of 0.5 by 0.5mm at the dermal-epidermal junction, follicular localization of atypical cells, and the finding of nucleated cells with the dermal papillae. The negative criterion included presence of broadened epidermal pattern. A score of 2 or more gave a sensitivity of 85% and a specificity of 76% for the diagnosis of LM (Table 1).
Diagnostic Algorithm of Lentigo Maligna of Guitera et al19
| Major Features (score +2 points) | Nonedged papillae |
| Pagetoid cells, round and greater than 20μm | |
| Positive minor features (score +1 point) | More than 3 atypical cells at the junction in 5 images |
| Follicular localization of pagetoid cells and/or atypical junctional cells | |
| Nucleated cells within the papilla | |
| Negative minor feature (score -1 point) | Broadened honeycomb pattern |
A score of more than 2 has a sensitivity of 85% and a specificity of 76% for the diagnosis of LM.
De Carvalho et al.23 retrospectively analyzed 60 pigmented macular facial lesions, including LM, LMM, SL/planar seborrheic keratosis, lichenoid keratosis, and pigmented actinic keratosis. Relating the dermoscopic features with RCM findings, they found that the fingerprint pattern was only found in benign lesions and corresponded in RCM to polycyclic papillary contours. The annular-granular pattern was present in both benign lesions and LM lesions. The blue and black rhomboid structures and the blue or black spots were seen almost exclusively in LM/LMM, and above all in nonincipient lesions. In a limited number of cases of benign lesions with spots or homogenous areas, the lesions corresponded to a cobblestone pattern in the epidermis and papillary polycyclic contours consisting of basal hyperpigmented cells located at the dermal-epidermal junction, typical of SL lesions and planar seborrheic keratosis. In LM/LMM, intraepidermal proliferation of dendritic cells was observed (a rare finding in benign lesions) with a tendency towards folliculotropism. In addition, the authors found junctional thickening comprised of atypical cells that were located radially around the follicles, adopting a tentacle-like appearance.
The utility of RCM in the follow-up of noninvasive treatments of LM was studied by Guitera et al.10 in 99 patients diagnosed with LM who had been treated with radiotherapy or imiquimod. Dermoscopy had a sensitivity of 80% and a specificity of 56%, whereas applying the diagnostic algorithm for LM with RCM, the specificity was 94% and the sensitivity 100%. The 2 dermoscopic signs that were most closely associated with histologic diagnosis of melanoma were asymmetric follicular openings and the presence of fine brown dots (similar to the dotted annular-granular pattern but without the annular distribution). This brown dotted pattern corresponded to the RCM finding of pagetoid cells, unlike the fine greyish points, which appeared as melanophages in RCM.
ConclusionsRCM is a very effective technique for the diagnosis of LM. The presence of atypical cells in the epidermis (with predominantly perifollicular localization) or the dermal-epidermal junction, as well as loss of normal skin architecture in the epidermal and dermal-epidermal junction are key for diagnosis of LM.
RCM permits us to analyze the entire surface of the lesion, as well as correlate findings with dermoscopic characteristics. RCM is an excellent technique for choosing areas to biopsy. It can delineate the margins of the lesion (even amelanotic lesions) and enable assessment of response to treatment and detection of recurrences in a noninvasive fashion.
We therefore believe that RCM is key not only in the diagnosis but also in the follow-up of LM.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gamo R, Pampín A, Floristán U. La microscopía confocal de reflectancia en el lentigo maligno. Actas Dermosifiliogr. 2016;107:830–835.