Probiotics, defined as live microbial dietary supplements that provide health benefits for the host, have been suggested as a treatment for atopic dermatitis based on a variety of proposed mechanisms of action. We analyzed evidence for the efficacy of probiotics to attenuate the severity of atopic dermatitis in pediatric patients younger than the age of 18 years.
Material and methodsSystematic review of trials of probiotics that included patients under the age of 18 years with a confirmed diagnosis of atopic dermatitis scored for severity using the Scoring Atopic Dermatitis SCORAD) tool. We performed a meta-analysis of the randomized placebo controlled trials. The following databases were searched: MEDLINE, Web of Science, Scopus, ClinicalTrials.gov, Epistemonikos, Trip Medical Database, and the Spanish Virtual Health Library.
ResultsTwenty trials were retrieved and included in the systematic review. Sixteen supported the use of probiotics to attenuate SCORAD-evaluated severity. Meta-analysis found an overall mean difference in effect between probiotics and placebo of −0.38 (95% CI, −0.63 to −0.14) in favor of probiotics. However, trial heterogeneity was high (I2 statistic, 76%) due to clinical and methodological variability.
ConclusionIn spite of clinical heterogeneity in trials attributable to different types of probiotic products and doses, and to the subjective variability of the SCORAD scale, we conclude that probiotics are beneficial for reducing the severity of atopic dermatitis as reflected by the SCORAD index.
Los probióticos, definidos como microorganismos vivos que proveen un beneficio para la salud del huésped, se han propuesto como una opción terapéutica para la dermatitis atópica (DA), habiéndose identificado varios mecanismos de acción. Se evaluó la eficacia del uso de probióticos para disminuir la gravedad de dermatitis atópica en pacientes pediátricos menores de 18 años.
Materiales y métodosSe realizó una revisión sistemática y metaanálisis que incluyó ensayos clínicos aleatorizados en pacientes menores de 18 años con diagnóstico establecido de dermatitis atópica, cuya gravedad estuvo medida por el Scoring Atopic Dermatitis (SCORAD) comparando el efecto de probióticos con el placebo, mediante la investigación en bases de datos MEDLINE, Web Of Science (WOS), Scopus, ClinicalTrials.gov, Epistemonikos, Trip medical database, Biblioteca virtual en salud (BVS).
ResultadosSe obtuvieron 20 estudios que fueron incluidos en la revisión sistemática, de los cuales 16 apoyan el uso de probióticos para reducir la gravedad del SCORAD en la dermatitis atópica. En el metaanálisis se obtuvo como resultado global una diferencia de medias de −0,38, con un IC 95% de −0,63 a −0,14, a favor del uso de probióticos; sin embargo, se encontró una alta heterogeneidad en los estudios debido a la variabilidad clínica y metodológica, con un I2 = 76%.
ConclusionesEl uso de probióticos es beneficioso para reducir la gravedad de la DA medida según SCORAD, a pesar de la presencia de una alta heterogeneidad clínica, derivada de sus diferentes tipos, dosis y variabilidad de una escala subjetiva como es el SCORAD.
Atopic dermatitis (AD) is a chronic, recurring disease of the skin, associated with abnormal barrier function of the skin, immunologic sensitization, and other mechanisms.1
The general prevalence of this disease has increased between 2-fold and 3-fold in recent decades. The disease manifests during the 1st year of life in approximately 60% of cases but may appear at any age. Its course may be continuous for long periods or may be recurrent. AD is mild in approximately 80% of children affected2.
Probiotics are defined by the WHO as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”3.
Multiple mechanisms have been proposed for how probiotics reduce atopy, including shifting the Th1/Th2 balance toward Th1 by inhibiting Th2 cytokines or increasing production of regulatory cytokines such as IL-10 through maturation of dendritic cells or their receptors4.
The objective of this study is to perform a systematic review and meta-analysis to determine whether oral administration of probiotics reduces the severity of atopic dermatitis based on the Scoring Atopic Dermatitis (SCORAD) scale in patients under 18 years of age with an established diagnosis of AD.
Materials and MethodsWe performed a systematic review that included randomized clinical trials published to 2020. The studies had to compare the effect of probiotics with a placebo in reducing the severity of atopic dermatitis. The study population was patients under 18 years of age with an established diagnosis of atopic dermatitis, the severity of which was measured using the SCORAD scale.
Studies that did not meet the inclusion criteria, inconclusive studies, those that presented conflicts of interest, narrative reviews, studies with results other than those sought, studies carried out on animals, studies with interventions other than the clinical interest of this study, and those with a population over 18 year of age were excluded.
The search was performed in the following databases: MEDLINE, Web Of Science (WOS), Scopus, ClinicalTrials.gov, Epistemonikos, Trip medical database, and Biblioteca virtual in salud (BVS). The search string was as follows: (((«Dermatitis, Atopic»[Majr]) AND «Probiotics»[Majr])) AND (((«Child»[Mesh]) OR «Child, Preschool»[Mesh]) OR «Adolescent»[Mesh]).
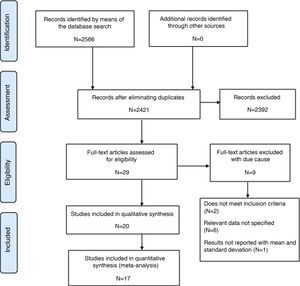
A total of 2566 articles were found and after eliminating duplicates, we obtained 2412 articles, of which, when the inclusion criteria were applied, 29 articles were obtained, and 2392 records were excluded. The authors MFT and AFPL evaluated the 29 articles based on their level of evidence and discarded 9: 2 were excluded because they did not meet the inclusion criteria, 6 did not specify important data, and 1 did not include the necessary statistical results. Finally, 20 studies were included in the systematic review and 17 studies in the meta-analysis (Fig. 1).
Statistical AnalysisThe information was extracted into databases using the programs Microsoft Excel and Review Manager (Rev Man), version 5.4. The results of studies were selected that made it possible to obtain the difference of means as a measure of the size of the continuous effect and its standard deviation with 95% confidence intervals (CI). Where possible, data was taken directly and in other cases, they were calculated using the Rev Man version 5.4 calculator. Clinical outcome was taken as the difference between the baseline SCORAD score and SCORAD score at the end of follow-up.
The χ2 test was used to determine heterogeneity, which was considered for P < .05, and the coefficient of inconsistency between studies (I2), considering heterogeneity if this coefficient is > 40%. The random effects model was used to carry out the meta-analysis and a funnel plot was drawn up to assess the risk of publication bias.5
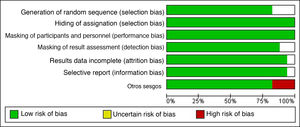
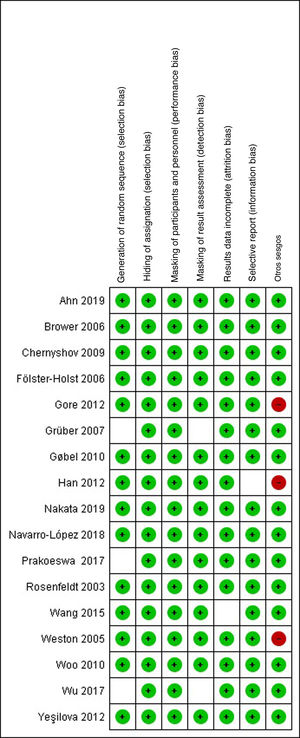
Risk-of-Bias AssessmentThe risk of selection, performance, detection, attrition, and notification bias was analyzed for each study based on the criteria proposed by the Cochrane Handbook for Systematic Reviews of Interventions. This analysis can be seen in Figs. 2 and 3, which show the individual risks of bias based on the criteria of both researchers.
Twenty studies included in the systematic review were analyzed (Table 1), of which 16 supported the use of probiotics to reduce the SCORAD severity in atopic dermatitis. With regard to the type of probiotic, 7 studies used combinations of strains and 13 used single strains; the most commonly used genus was Lactobacillus, species rhamnosus. The frequency of administration of the probiotic varied from 1 to 2 times per day and duration of treatment in the studies varied between 4 and 12 weeks.
Studies Included.
| Study | Population | Probiotics (Genus, Species, Strain) | Duration | Study Conclusion | |
|---|---|---|---|---|---|
| 1 | Navarro-López et al.4, 2018 | 47 children aged 4 to 17 years with AD, moderate SCORAD | Bifidobacterium lactis CECT 8145, B longum CECT 7347, and Lactobacillus casei CECT 9104 | 12 wk | Use of probiotics is useful in reducing SCORAD |
| 2 | Prakoeswa et al.6, 2017 | 22 children aged 0 to 14 years with AD | Lactobacillus plantarum IS-10506 | 12 wk | The use of probiotics is a potential treatment for AD in children |
| 3 | Wang y Wang7, 2015 | 212 children aged 1 to 18 years AD and at least 1 positive skin prick or IgE antibody test specific to common allergens | Lactobacillus paracasei GMNL-133, Lactobacillus fermentum GM090 | 12 wk | The mix of probiotics is associated with clinical improvement of AD |
| 4 | Wu et al.8, 2017 | 67 children aged 4 to 48 months AD, SCORAD ≥ 15 | Lactobacillus rhamnosus (MP108) | 8 wk | The use of probiotics reduces all SCORAD parameters in children with AD |
| 5 | Han et al.9, 2012 | 83 children aged 1 to 13 years with AD, SCORAD between 20 and 50 | Lactobacillus plantarum CJLP133 | 16 wk | Probiotic supplements are beneficial for the treatment of AD |
| 6 | Gore et al.10, 2012 | 137 children aged 3 to 6 months with AD, SCORAD ≥ 10 | Lactobacillus paracasei CNCM I-2116 or Bifidobacterium lactis CNCM I-3446 | 12 wk | No benefit was found for the use of probiotics in the treatment of AD |
| 7 | Woo et al.11, 2010 | 75 children aged 2 to 10 years with AD, SCORAD > 25 | Lactobacillus sakei KCTC 10755BP | 14 wk | The use of probiotics is associated with clinical improvement of AD in children |
| 8 | Weston12, 2005 | 53 children with a diagnosis of AD and SCORAD ≥ 25 | Lactobacillus fermentum VRI-033 PCC | 16 wk | The probiotic supplement is beneficial in reducing the extension and severity of SCORAD |
| 9 | Sistek et al.13, 2006 | 49 children aged 1 to 10 years and at least 1 positive skin prick or specific IgE antibody test, or a RAST test positive for common allergens | Lactobacillus rhamnosus and Bifidobacterium lactis | 18 wk | Probiotics improved clinical signs and symptoms of AD only in patients with food sensitivities |
| 10 | Grüber et al.14, 2007 | 102 children aged 3 to 12 months with AD, moderate to severe SCORAD | Lactobacillus rhamnosus GG | 12 wk | No therapeutic effect for reducing the severity of AD was found |
| 11 | Brouwer et al.15, 2006 | 50 children aged under 5 months with AD | Lactobacillus rhamnosus (NP-Lrh), Lactobacillus GG (NP-LGG) | 12 wk | No statistically significant effect of probiotic supplements on SCORAD, inflammatory parameters, or cytokine production |
| 12 | Fölster-Holst et al.16, 2006 | 54 children aged 1 to 55 months with moderate to severe AD | Lactobacillus rhamnosus strain GG (LGG) | 8 wk | No significant differences were found between the groups in terms of clinical symptoms (SCORAD, pruritus, sleep loss) |
| 13 | Rosenfeldt et al.17, 2003 | 43 children aged 1 to 13 years with AD | Lyophilized Lactobacillus rhamnosus (19070-2) and Lactobacillus reuteri (DSM 122460) | 6 wk | A combination of probiotics was beneficial in the treatment of AD |
| 14 | Yeşilova et al.18, 2012 | 40 children aged 1 to 13 years with AD | Bifidobacterium bifidum, Lactobacillus acidophilus, L casei, L salivarius | 8 wk | The use of probiotics is effective in reducing the SCORAD score in patients with AD |
| 15 | Nakata et al.19, 2019 | 59 children aged 10 months to 3 years with AD | Lactobacillus acidophilus L-92 (L-92) | 24 wk | Consuming a specific quantity of L-92 functions as a complementary treatment for AD |
| 16 | Han et al.9, 2012 | 124 children aged 2 to 13 years with mild to moderate AD | Lactobacillus pentosus | 4 wk | The SCORAD scores for the probiotics group improved significantly compared to the placebo group in AD |
| 17 | Gøbel et al.20, 2010 | 50 children aged 7 to 24 months with AD | Lactobacillus acidophilus NCFM, Bifidobacterium animalis ssp. lactis Bi-07 | 8 wk | No clinical or general immunologic effect was shown by probiotic supplements in children with AD |
| 18 | Chernyshov21, 2009 | 58 children aged 2 months to 4 years with AD | Lactobacillus rhamnosus R0011, L helveticus R0052 | 4 wk | The use of the probiotic Lactobacillus acidophilus is associated with a larger number of patients who achieved marked clinical improvement and a greater corticosteroid-saving effect |
| 19 | Isolauri et al.22, 2000 | 27 children with a mean age of 4.6 months with AD | Bifidobacterium lactis Bb-12, Lactobacillus strain GG | 8 wk | A significant improvement occurred in the general condition of the skin in patients who received formulae supplemented with probiotics, compared to the non-supplemented group |
| 20 | Viljanen et al.23, 2005 | 230 children aged 1.4 to 11.9 months with AD | Lactobacillus GG (LGG), L rhamnosus LC705 (LC705), Bifidobacterium breve Bbi99, Propionibacterium freudenreichii ssp. shermanii JS (Propionibacterium JS) | 4 wk | Treatment with LGG shows a greater reduction of the SCORAD score than the placebo group in infants suffering from AD associated with IgE |
Abbreviations: B indicates Bifidobacterium; AD, atopic dermatitis; IgE, immunoglobulin E; L, Lactobacillus; SCORAD, Scoring Atopic Dermatitis.
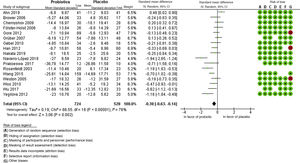
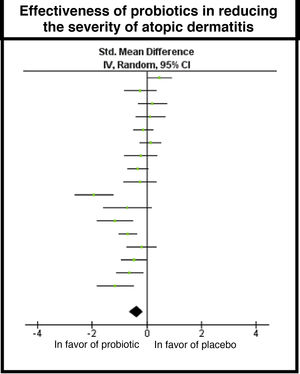
The meta-analysis included 17 studies and the global result found a difference in measurement of –0.38, with a 95% CI of –0.63 to –0.14, in favor of the use of probiotics; however, high heterogeneity was found in the studies due to clinical and methodological variability, with I2 = 76% and χ2 with P = .00001 (Fig. 4).
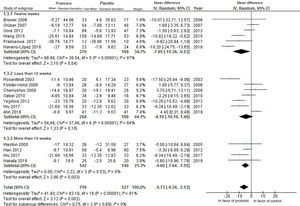
Subgroup analyses were performed according to type of strain (Fig. 5) and follow-up time (Fig. 6) for the use of probiotics to reduce the SCORAD; nevertheless, high heterogeneity was found in both analyses. With regard to the strain, greater benefit was not found with the use of Lactobacillus rhamnosus in comparison with the other probiotic strains. Based on the time of administration, a greater benefit was found after administration of probiotics for 12 weeks.
A sensitivity analysis was performed, and no variations were found after repeatedly reviewing the data, and the results suggested no important changes. Reporting bias was assessed using a funnel plot (Fig. 7) and was corroborated using the trim-and-fill method24, where a symmetric distribution of the studies can be observed, approaching a funnel shape, which makes it possible to rule out publication bias.
DiscussionThe results of this systematic review indicated that probiotics have a beneficial effect in pediatric patients with atopic dermatitis.
These results are supported by the results obtained in the meta-analysis, in which the global results support the use of probiotics to reduce the SCORAD. The heterogeneity of the studies, however, does not make it possible to determine the real magnitude of the degree to which the probiotics reduce the severity of AD as measured by SCORAD.
This heterogeneity is due to the different interventions in each study, resulting from the use of different strains, the doses of probiotics, different duration of the treatments, and the subjectivity of the SCORAD scale for evaluating the severity of the AD.
Given the heterogeneity of the characteristics of the populations between the different studies, the analysis by subgroups does not show potentially real results, except for follow-up, in which case it was established that the efficacy of the use of probiotics is observed following administration for more than 12 weeks.
Makrgeorgou et al25 performed an update of a systematic review similar to ours, based on a previous review carried out in 2017. That review included 39 articles that, although they provided a significant sample, did not specifically analyze the pediatric population and included the use of symbiotics. The study concluded that probiotics reduce SCORAD severity but with a very low score for determining a significant change in the symptoms of atopic dermatitis.
The systematic review carried out by Huang et al26 found that more evidence was required to recommend probiotics in a generalized manner. This is because the articles included covered small and heterogeneous populations, which reduced the power of evidence of the review, unlike this review, which includes a larger number of studies and with larger populations.
The limitations of this systematic review include the use of different strains of probiotic, which may lead to different results and individual conclusions in each study. Moreover, several major differences exist between the participants in the clinical trials: in some, it was established that the participants should follow a strict diet with no fermented foods, restriction of the use of antibiotics, and other requirements that were not present in all the clinical trials.
In the statistical analysis for the meta-analysis, heterogeneity was found between the populations of the different studies. This constituted a limitation when analyzing the results, as it made it impossible to determine the reduction in the SCORAD score expected in patients with AD following administration of the probiotic. Another limiting factor involves the association of pharmaceutical companies and food manufacturers in the manufacture of probiotics for the trials, which may be a potential source of conflicts of interest.
The use of probiotics has been studied for several years, with a particular focus on diseases linked to the digestive system. Given that a wide range of probiotics exists on the market in our country, we may begin to use them as coadjuvant treatment in AD.
ConclusionOral probiotics, especially strains of bacteria of the genus Lactobacillus, are useful for reducing the severity of atopic dermatitis but more homogeneous studies are required to determine the magnitude of this benefit.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors would like to thank the Pontificada Universidad Católica del Ecuador for encouraging this study.
Please cite this article as: Pachacama López AF, Tapia Portilla MF, Moreno-Piedrahíta Hernández F, Palacios-Álvarez S. Uso de probióticos para disminuir la gravedad de la dermatitis atópica en población pediátrica: revisión sistemática y metaanálisis. Actas Dermosifiliogr. 2021;112:881–890.