Patients with cutaneous metastatic melanoma of unknown primary origin (stage IV M1a disease according to the American Joint Committee on Cancer melanoma staging system) have an estimated 5-year survival rate of between 5% and 17.9% and a median survival of 6 months. However, certain patients with stage IV M1a disease have much higher survival rates. The existence of this subpopulation has given rise to the term primary dermal melanoma to describe such cases.
We report a case of melanoma with characteristics consistent with primary dermal melanoma and review the relevant literature. A diagnosis of primary dermal melanoma requires careful clinical and pathologic correlation and should be considered in all patients with a solitary melanoma confined to the dermis and subcutaneous tissue when there is no evidence of a primary tumor or disease at other sites following appropriate staging studies. We believe that familiarity with this subtype of melanoma is essential in order to provide patients with optimal care and better prognostic information.
La supervivencia de pacientes con metástasis cutánea de melanoma de origen primario desconocido, clasificados como estadio IV (M1a) por el American Joint Comittee on Cancer para melanoma, se estima en un 5–17,9% a los 5 años, con una mediana de 6 meses. Es conocida la existencia de pacientes así clasificados que presentan una supervivencia mucho mayor, lo que ha llevado a utilizar el término de «melanoma dérmico primario» (MDP).
Presentamos un caso compatible con MDP, así como una revisión de los principales artículos publicados. El diagnóstico está sujeto a una correcta correlación clínico-patológica y debe ser considerado en todos los pacientes con melanoma solitario confinado en la dermis y en el tejido celular subcutáneo, en los que no se encuentre un origen primario ni evidencia de enfermedad tras un adecuado estudio de extensión. Creemos necesario el conocimiento de esta posibilidad para un correcto manejo e información pronóstica de los pacientes.
The term primary dermal melanoma (PDM) has been used to describe a subtype of melanoma which is confined to the dermis or to the subcutaneous cellular tissue and histologically simulates metastasis, but is nonetheless associated with an unexpectedly long survival.1,2
In view of the controversy surrounding the origin of solitary foci of melanoma in the dermis or subcutaneous cellular tissue, some authors have preferred to continue using the term solitary dermal melanoma (SDM), although those authors have also reported a longer survival, similar to that of intermediate-thickness primary melanomas.3
We present the case of a 76-year-old woman with a PDM mimicking an apocrine hidrocystoma. We also review the most relevant articles on this subject (MEDLINE search, June 2011), though we found no reports in the Spanish-language literature.
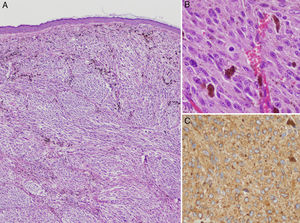
Case DescriptionThe patient was a 76-year-old woman referred to dermatology outpatients for a lesion that had been present for 7 years on the left lower eyelid, adjacent to the malar region. The lesion had shown slow and progressive growth that had accelerated in the previous 6 months. There were no systemic symptoms. Physical examination revealed a mobile, well-defined, translucent bluish-gray nodule of 1.2cm diameter with no changes in the overlying epidermis. Apocrine hidrocystoma was diagnosed and the lesion was excised. Histology revealed a nodular lesion that reached the reticular dermis. The cytological and immunohistochemical characteristics were consistent with melanoma metastasis with a Breslow thickness of 9mm. Histology of multiple serial sections showed no signs of ulceration or regression and no junctional component (Fig. 1).
A, Clearly defined nodular lesion in the dermis, separated from the epidermis by a Grenz zone. The nodule is composed of atypical melanocytes and shows no signs of regression, ulceration, or any epidermal component (hematoxylin-eosin, original magnification ×200). B, Detail showing atypical cells and mitotic figures (mitotic index, 2/mm2) (hematoxylin-eosin, original magnification ×400). C, HMB45 stain.
Complete physical examination, including examination of the eyes and mucosas, revealed no lesions suggestive of malignancy, no palpable lymph nodes or organomegaly, and no areas of leukoderma. The patient said that she had not had any previous pigmented lesions that had been excised or that had undergone spontaneous regression.
The results of a full range of laboratory tests, including lactate dehydrogenase, were completely normal, and positron emission tomography showed no areas of pathological uptake.
DiscussionIn approximately 2% to 5% of melanoma metastases there is no known primary tumor; two thirds of these metastases are lymphatic and a third affect the skin and/or subcutaneous cellular tissue or other organs.4 These latter forms are classified as stage IV (M1a in the case of skin metastases) by the American Joint Committee on Cancer (AJCC) for melanoma, and they are associated with an estimated 5-year survival of 5% to 17.9%.4
The incidence of solitary melanoma lesions confined to the dermis and/or subcutaneous cellular tissue is less than 1% in all series.1,3–8 However, survival rates of 71% to 100% have been reported after follow-up periods of 4 to 8 years, except in the series published by Katz et al.,8 in which the estimated 5-year survival was 25% (though those authors did not specify whether all their patients had solitary dermal lesions) (Table 1).
Site, Mean Age, Treatment, Incidence, and Survival of Patients With Solitary Dermal Melanoma or Primary Dermal Melanoma.
| Reference | No. of Cases | Site | Age, y (Range) | Treatment | Total No. of Melanomas | Incidence | Survival |
|---|---|---|---|---|---|---|---|
| Giuliano et al.6 | 5 | _ | 43.0a (20–70) | Variable (resection, chemotherapy, radiotherapy or immunotherapy) | 980 | 0.92% | 80% (5 y) |
| Schlagenhauff et al.5 | 30 | Limbs (woman)Head and neck (both)Trunk (man) | 53 (15–85) | Excision with 1–2cm margin | 3258 | 0.92% | 83% (5 y) |
| Anbari et al.7 | 3 | Trunk (2)Thigh (1) | 49.5 (29–74) | Surgical excision | – | – | 100% (4 y) |
| Bowen et al.4 | 11 | Back (3)Limbs (5)Head (2)Neck (1) | 55.7 (28–90) | Excision with a 1–2cm margin (11)Interferon alfa (1) | 1800 | 0.61% | 83% (8 y) |
| Swetter et al.1 | 7b | Head (4)Limbs (3) | 67.4 (22–85) | Wide local excision (7)Interferon alfa (2)Sentinel lymph node biopsy: 6 negative, 1 lost | 1800 | 0.39% | 100% (5 y) |
| Katz et al.8c | 12 | - | - | 2485 | 0.48% | 25% (5 y) | |
| Cassarino et al.2 | 13 (6+7b) | Head (7)Limbs (5)Back (1) | 70 (21–85) | Excision with a 2cm margin (9)Sentinel lymph node biopsy in 11: 10 negative, 1 lost | – | – | 92% (44 mo) |
| Lee et al.3 | (85d) 71e | Head and neck (13)Trunk (27)Limbs (31) | <60 (50)>60 (21) | Wide local excisionSentinel lymph node biopsy in 20: 3 positiveElective lymphadenectomy in 17: 4 positive | 12 817 | <1% | 73% (5 y) |
| González de Arriba et al. | 1 | Head | 76 | Limited excision | – | – | 8 y |
Bowen et al.4 described 11 patients with solitary melanomas confined to the dermis and/or subcutaneous cellular tissue (patients with a past history of melanoma or with surgical excision or regression of cutaneous or ocular lesions suggestive of melanoma were excluded). Histologically the tumors were well-defined nodules composed of atypical melanocytes, with no junctional component or signs of regression. In that case series the estimated survival at 8 years was 83%, considerably higher than expected, but similar to previous studies.5–7 Those authors finally concluded that the foci of melanoma in their patients were primary tumors that arose in the dermis or subcutaneous cellular tissue from nonepidermal remnants or aberrations of embryologic melanocyte migration or melanocytes included in deep adnexal structures.
Later, Swetter et al.1 used the term PDM to refer to patients with solitary melanoma in the dermis and/or subcutaneous cellular tissue and proposed a new subtype of melanoma with an excellent prognosis, perhaps even less aggressive than primary melanomas of similar thickness, with a mean Breslow thickness of 6 to 7mm.4
Lee et al.3 reported the largest series, including 12 817 patients with melanoma. More than 900 of their patients had melanoma without a known primary, and 101 of these tumors presented as dermal melanomas: 85 as localized disease, 7 with regional involvement, and 9 with metastatic disease at the time of diagnosis. The majority of patients with SDM in that study were less than 60 years of age, most were men, and the tumors arose mainly on the limbs; our patient did not fall into any of these categories. Patients with SDM in that study also had a better prognosis, with an estimated 5-year survival of 73%, similar to the survival in thick (Breslow thickness, 6–7mm) or intermediate-thickness (Breslow thickness, 1–2mm) primary melanomas. It is particularly noticeable that 23% of patients with SDM can present lymph-node involvement at the time of diagnosis or later. These patients would therefore benefit from sentinel lymph node biopsy, followed by lymphadenectomy when applicable, with management similar to that used in regional disease without distant spread. Patients with SDM and lymph-node involvement have an estimated 5-year survival of 67%, similar to or better than the survival in primary melanomas with lymph-node metastases (classified as stage III in the AJCC melanoma staging system), which is between 13% and 50%.
The origin of PDM is controversial. The following possibilities have been proposed: a) a distant or in-transit skin metastasis from a primary melanoma that has regressed completely, b) a primary nodular melanoma or a melanoma whose junctional component has regressed, and c) a true primary dermal melanoma. Based on the findings of survival studies, these tumors are unlikely to be melanoma metastases, as survival is closer to the estimates for thick or intermediate-thickness primary melanomas.3 Cassarino et al.2 suggested that immunohistochemical characterization of PDM shows lower levels of expression of p53, Ki-67, cyclin D1, and D2-40 than found in cutaneous melanoma metastases or primary nodular melanoma, and they considered that these differences would explain the less aggressive biological behavior of PDM.
PDM is difficult to recognize clinically. It can present as a cystic lesion, a violaceous papule, a bluish or grayish subcutaneous nodule, or a poorly defined subcutaneous mass. In the differential diagnosis we should consider basal cell carcinoma, squamous cell carcinoma, dermatofibroma, neurofibroma, hemangioma, amelanotic melanoma, and adnexal tumors,1,2 including apocrine hidrocystoma, as suspected in the case we present.
The histology findings show a well-defined nodular or multinodular dermal lesion with cellular atypia, numerous mitoses, and areas of necrosis, with no evidence of an in situ or junctional component, no follicular involvement or neural invasion, no ulceration, regression, or vascular or lymphatic invasion, and no history of a previous melanocytic nevus. The Breslow thickness varies between 2.5 and 11.7mm, with a mean of 7mm.1 The present case fitted all these descriptions.
The histopathological differential diagnosis includes metastatic melanoma, nodular melanoma, malignant blue nevus, clear cell sarcoma of tendons and aponeuroses (also known as malignant melanoma of soft parts), and malignant peripheral nerve sheath tumor. Malignant blue nevus is a lesion that clearly arises from a pre-existing blue nevus and it has a very poor prognosis. In other tumors, immunohistochemistry can help to confirm or exclude the diagnosis.4 Clear cell sarcoma is characterized by the reciprocal translocation t(12;22)(q13;q12), which gives rise to the EWS-AFT1 fusion protein, present in 70% to 90% of cases.9,10 Malignant peripheral nerve sheath tumor is more common in patients with neurofibromatosis and affects large peripheral nerves. This tumor can be partially encapsulated and shows areas of neural differentiation associated with other areas of myxoid stroma.2
The diagnosis of PDM must be based on a correct correlation of the clinical and pathological findings. Workup must therefore include a detailed history to detect possible skin lesions that have regressed or have been excised, a meticulous physical examination (including all mucosas and ophthalmic and gynecological examination), screening for tumor spread (including biochemistry with lactate dehydrogenase, positron emission tomography and/or computed tomography, and cranial magnetic resonance imaging),1 and sentinel lymph node study for correct staging.3 These patients will benefit from wide local excision combined, when indicated, with lymphadenectomy.
In our case, limited excision of the lesion was performed without extending the surgical margins and without sentinel lymph node biopsy because of the location of the lesion on the face and following the wishes of the patient. After a year of follow-up, 8 years since the lesion first appeared, the patient remains asymptomatic, with no signs of disease recurrence or progression. The diagnosis of PDM was based on clinical and histological findings and on the clinical course. We would like to draw attention to the fact that, as the growth of the lesion was very slow, late metastases are possible, as the growth rate of metastases appears to be related to the growth rate of the primary tumor.11
In conclusion, we have presented a case that supports the diagnosis of PDM as a variant of melanoma. PDM carries a better prognosis than might be expected on the basis of its histological characteristics and Breslow thickness. This diagnosis should be considered in any patient with a solitary melanoma confined to the dermis or subcutaneous cellular tissue, with no evidence of a primary origin or of distant spread after adequate screening. We believe it is necessary to be aware of this type of melanoma in order to provide optimal management and correct prognostic information for these patients.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: González-de Arriba M, et al. Melanoma dérmico primario: presentación de un caso y revisión de la literatura. Actas Dermosifiliogr. 2013;104:518–22.