To review the literature on validated tools for measuring patient-reported outcomes (PROs) in psoriasis in Spain. To evaluate the psychometric properties of the tools and describe the results of their practical application.
Material and methodsSystematic review of studies validating or using instruments for assessing PROs in Spanish patients with psoriasis. Literature searches were performed in international (PubMed/Medline) and Spanish (Medes, Ibecs) databases. We also searched databases of instruments for measuring PROs (BiblioPRO, PROQOLID). The review included studies published in English or Spanish up to January 9, 2017. We also checked the reference lists of the key publications identified. The quality of the questionnaires was evaluated based on their psychometric properties (construct, transcultural adaptation, reliability, validity, feasibility, and sensitivity to change).
ResultsEighteen publications were included. Six articles described the validation of Spanish versions of 5 PROs tools: 4 health-related quality of life (HRQoL) questionnaires specific to psoriasis and dermatologic diseases and 1 questionnaire specific to satisfaction with treatment. Our assessment of the HRQoL tools’ psychometric properties showed that the PSO-LIFE questionnaire received the highest scores, although specific properties varied from instrument to instrument. The 12 remaining articles were observational studies that used the validated instruments. In use, these tools detected the high impact of psoriasis on HRQoL, especially in young female patients with severe disease.
ConclusionsWe identified 5 specific instruments validated in Spain for scoring PROs in patients with psoriasis. The tools’ psychometric properties vary, and it is essential to understand their strengths and weaknesses when selecting the right one for each situation. In use, these questionnaires are able to detect the high impact of psoriasis on patients’ HRQoL. PROs provide useful information to complement routine clinical findings in psoriasis and may contribute to improving disease management.
Revisar la bibliografía sobre instrumentos específicos de medida de los resultados percibidos por los pacientes (patient reported outcomes, PRO) validados y utilizados en población española con psoriasis, valorar sus propiedades psicométricas y describir los resultados de su aplicación práctica.
Materiales y métodosRevisión sistemática de la literatura científica en bases de datos internacionales (PubMed/Medline) y nacionales (Medes, Ibecs) referente a estudios que validen o implementen instrumentos específicos de valoración de PRO en población española con psoriasis. Se completó la búsqueda en bases de datos específicas de instrumentos para medir PRO (BiblioPRO, PROQOLID). Se incluyeron los estudios publicados en inglés o español hasta el 01/09/2017. Adicionalmente, se revisaron las listas de referencias bibliográficas de las publicaciones clave identificadas. La valoración de la calidad metodológica de los cuestionarios se efectuó con base en sus propiedades psicométricas (constructo, adaptación transcultural, fiabilidad, validez, factibilidad y sensibilidad al cambio).
ResultadosSe seleccionaron 18 publicaciones. Seis artículos describieron la validación al español de 5 instrumentos de PRO: 4 cuestionarios de calidad de vida relacionada con la salud (CVRS) específicos de psoriasis/enfermedades dermatológicas y un cuestionario específico de satisfacción con el tratamiento. La valoración psicométrica muestra variabilidad en los criterios alcanzados por cada instrumento; el cuestionario de CVRS PSO-LIFE resultó el más completo. Los 12 artículos restantes correspondían a estudios observacionales que empleaban los instrumentos validados. Su utilización muestra un elevado impacto de la psoriasis en la CVRS, especialmente en pacientes jóvenes, de género femenino y con enfermedad grave.
ConclusionesSe han identificado 5instrumentos específicos validados en España para valorar los PRO en pacientes con psoriasis. Dada la variedad de sus propiedades psicométricas, resulta esencial conocer las fortalezas y debilidades de cada uno para seleccionar el instrumento apropiado para cada situación. El empleo de estos cuestionarios pone de manifiesto el elevado impacto de la enfermedad en la CVRS de los pacientes. La evaluación de los PRO en el paciente con psoriasis complementa los resultados clínicos tradicionales y puede contribuir a un manejo más óptimo de la enfermedad.
Psoriasis is a chronic inflammatory skin disease that can substantially alter patient quality of life2 and affect both physical and psychological well-being.1
Clinical measures such as Psoriasis Area and Severity Index (PASI), Physician Global Assessment (PGA), and body surface area (BSA) scores are typically used to assess disease severity and treatment effectiveness. They do not capture, however, patient perspectives of how their lives are affected by psoriasis,1 highlighting the need to incorporate patient-reported outcomes (PROs) in patient assessments. Several tools exist to assess PROs in psoriasis, but there are no standardized criteria on when each of them should be used.
Health-related quality of life (HRQoL) is the PRO that has attracted most interest in recent decades and it can be evaluated using a wide range of generic questionnaires (applicable to different populations) and disease-specific questionnaires designed to overcome the lack of sensitivity that characterizes more general tools.3 Tools to evaluate other PROs, such as patient satisfaction or expectations, are also gaining interest. Few studies to date, however, have analyzed PROs in psoriasis and there is also a shortage of disease-specific questionnaires in this setting.4
The measurement properties of any new PRO assessment tool must be validated prior to use.3 In addition, before being applied to a new cultural setting (e.g., a new country), existing tools must first be modified using a systematic, standardized cross-cultural adaptation process and then validated.
The aims of this study were to review the literature on specific PRO assessment tools validated or used in Spanish patients with psoriasis, to assess their psychometric properties, and describe the findings of studies that have used these tools.
MethodsWe performed a systematic review of the literature to identify studies that have validated or used specific PRO assessment tools for psoriasis in Spain. We searched both Spanish (Medes, Ibecs) and international (PubMed/MEDLINE) databases up to September 1, 2017 following the recommendations set out in the Cochrane Handbook for Systematic Reviews of Interventions (Supplementary Table 1). We also searched specific PRO research tool databases (BiblioPRO, PROQOLID) and reviewed the reference lists of key articles retrieved in our search.
To be included, studies had to 1) describe the validation or use of a generic PRO assessment tool for patients with skin diseases (including psoriasis) or a psoriasis-specific tool and 2) have been used in a population in which at least 50% of the patients were Spanish.
Two researchers working separately selected the articles for inclusion and evaluated the questionnaires’ psychometric properties. Discrepancies during the selection or evaluation process were resolved by consensus with the involvement of a third researcher.
The methodologic quality of the validated questionnaires was assessed by analyzing their psychometric properties in accordance with the recommendations of the US Food and Drug Agency,5 EMPRO,6 and the Medical Outcomes Trust.7 To facilitate evaluation without compromising accuracy, we studied the 6 most relevant properties: conceptual model and measurement (3 items), cross-cultural adaptation (3 items), reliability (2 items), validity (4 items), feasibility (4 items), and sensitivity to change (2 items) (Supplementary Table 2). The reviewers rated item compliance and suitability on a 4-point scale: totally agree (+++), agree quite a lot (++), agree a little (+), and totally disagree or no evidence available (Ø). The total score assigned to each questionnaire was calculated by adding up the partial scores for each item (+=1 point) and converting these to scores on a scale of 0 to 100, with higher scores indicating better psychometric properties.
The Oxford Centre for Evidence-Based Medicine guidelines were used to classify the level of evidence for each study.8
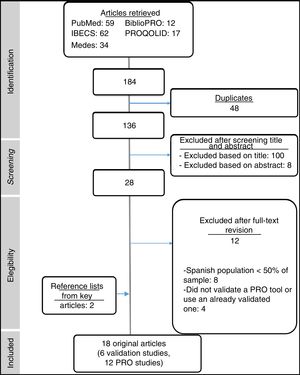
ResultsOur initial search retrieved 184 articles, 16 of which were considered relevant to the study. An additional 2 articles were identified during our search of key reference lists (Fig. 1). Of the 18 articles included in the final sample, 6 were validation studies of PRO assessment tools for use in Spain (Table 1) and 12 were observational studies that had used these tools in the Spanish population (Table 2). The 12 articles excluded after applying the selection criteria are shown in Supplementary Table 3.
Description of Studies That Have Validated Patient-Reported Outcome Tools in Spain.
| Tool, Author (Year), Study Design, and Level of Evidence | Description of Sample | HRQoL Variables Analyzed | [0.4-5]Results | General Conclusions | |
|---|---|---|---|---|---|
| Specific tools for measuring HRQoL in psoriasis | |||||
| PSO-LIFEDauden et al.19 (2012)Prospective, observational, multicenter2C | 304 patients with active or stable psoriasis and 56 with urticaria or atopic dermatitis (control group)Mean (SD) age: 45.3 (14.5) y in psoriasis group vs 38.8 (14) y in control group (P<.01)% of male patients: 56.3% in psoriasis group vs 51.8% in control group | HRQoL: PSO-LIFE, DLQI, PDIClinical: PASI | Conceptual model | - Clear description of concept being measured.- Literature review to explore association between psoriasis and patient-perceived HRQoL.- Use of an expert focus group and patient interviews to identify domains and select determinants of HRQoL in patients with psoriasis.- Preliminary patient analysis. | Results showed that the PSO-LIFE questionnaire is reliable, valid, and sensitive to change in the target population. |
| Cross-cultural adaptation | - New questionnaire developed for Spanish patients with psoriasis. | ||||
| Reliability | - Good internal consistency (Cronbach α, 0.95).- High reproducibility (ICC, 0.98). | ||||
| Validity | Content- High response rate.- Scoring of items by expert panel.- Preliminary factor analysis with patients.Construct- Factor analysis: unidimensional questionnaire.CriterionConvergent- Differences in PSO-LIFE scores between psoriasis patients and controls (P<.03), active and inactive psoriasis (P<.01), location of lesions (P<.01), mild and moderate disease (P<.01), and patients with inactive psoriasis and control group (P<.05).- Moderate correlation between PASI and PSO-LIFE scores (r=−0.43, P<.01).- Moderate to high correlation between PSO-LIFE and DLQI and PDI scores (−0.4 to −0.8).Longitudinal- Correlation between changes in PASI and PSO-LIFE scores from baseline to end visit at 3 mo (r=−0.4, P<.01).- Moderate to high correlation between changes in PSO-LIFE and DLQI and PDI scores (r=−0.69 and r=−0.67, respectively. | ||||
| Feasibility | Floor effect, 0%; ceiling effect, 3.2%- Low rate of unanswered items (5%). | ||||
| Sensitivity to change | - Mean effect size of 0.5; 0.73 in patients with active psoriasis and 0.29 in patients with inactive psoriasis.- Minimally clinically important difference at 3 mo: approximately 6.5 points (calculated using results for patients who reported “slightly improved” health at 3 mo. | ||||
| PDIVanaclocha et al.3 (2005)Prospective, observational, multicenter2C | 294 patients with moderate or severe psoriasisMean (SD) age: 43.1 y% of male patients: 58% | HRQoL: PDI and EQ-5D (descriptive system and VAS), patient- and researcher-perceived psoriasis severityClinical: PASI | Conceptual model | - Clear description of concept being measured.- Description of domains and number of items per domain. | The Spanish version of the PDI has good psychometric properties in terms of reliability, validity, feasibility, and sensitivity to change. |
| Cross-cultural adaptation | - Translation-backtranslation.20 | ||||
| Reliability | - Good internal consistency (Cronbach α, 0.89). | ||||
| Validity | CriterionConvergent- Higher PDI scores in patients with higher PASI scores (more severe disease) (r=0.33, P<.01).- Correlation between PASI and PDI (r=0.33) and VAS (r=−0.41) scores (P<.01).- Correlation between PDI and EQ-5D scores (P<.05).- Correlation between more severe psoriasis as perceived by physicians and patients and greater impact on HRQoL (higher PDI scores) (P<.001).Longitudinal- Correlation between changes in PDI scores from baseline to end visit at 6 mo and PASI and VAS scores (r=0.39 and –0.51, P<.001). | ||||
| Feasibility | - 87.1% of patients completed the full questionnaire; 98.6% answered >80% of the questions. | ||||
| Sensitivity to change | - Adequate effect size (0.95) | ||||
| Specific instruments for measuring HRQoL in skin diseases | |||||
| DLQIBadia et al.21 (1999)Observational study2C | 237 patients with eczema (48%) or psoriasis (52%) and 100 individuals from the general population; 143 patients initiating treatment and 94 with stable diseaseMean (SD) age: 40.3 y in eczema group, 38.7 in psoriasis group, and 45.4 in general population- % of male patients: 42.1% in eczema group, 51.2% in psoriasis group, and 32.3% in general population | Disease-related: clinical severity (assessed on 4-point scale: absent, mild, moderate, severe)Tools: DLQI and NHP | Cross-cultural adaptation22 | - Translation-backtranslation and piloting.- Agreement between translators and author of original tool. | The Spanish version of the DLQI had a substantial floor effect and lacked sensitivity to change in most domains.The emotional reactions and mobility domains in the NHP were more responsive than some DLQI domains. It is probably advisable to use generic tools in addition to the DLQI. |
| Reliability | - Good internal consistency (Cronbach α, 0.83).- Good reproducibility (ICC, 0.88). | ||||
| Validity | Content- Proportion of irrelevant answers: 5% (patients).Construct- Comparison of scores between patients with skin diseases and the general population: significantly higher scores in patients (4.3 vs 0.25, P<.001).CriterionConvergent- Correlations between DLQI scores, clinical severity measures, and NHP domain scores. The correlations were very weak, but significant (P<.001). Correlations with NHP domains and clinical measures: 0.12 to 0.32 and 0.26, respectively. | ||||
| Feasibility | - Very high floor effect for most domains: acceptable (7%) for “perceptions” and high (53%-80%) for others.- Just 2% of patients did not answer 1 or more items.- Patients took a mean (SD) of 7.6 (4.8) min to complete the questionnaire. | ||||
| Sensitivity to change | - Effect size for patients starting treatment: 0.70. Large effect size (0.82) for patients with eczema and moderate size (0.58) for those with psoriasis. The effect, however, was small (range, 0.03-0.35) for most domains. A large effect (1.0) was only observed for the symptoms and perceptions domain. | ||||
| Skindex-29Jones-Caballero et al.23 (2000): translation, adaptation, and preliminary validationObservational2C | 103 people with and without skin disease (41 with psoriasis or eczema)Mean (SD) age: 40 (16) y% of male patients: 31% | Tool: Skindex-29 | Conceptual model | - Clear description of concept being measured.- Description of domains and number of items per domain.- Translation and cross-cultural adaptation.- Patients involved in item selection. | The preliminary evaluation suggests that the Spanish version of Skindex-29 is semantically equivalent, reliable, and valid. Studies demonstrating that this version is reproducible and equivalent are needed to confirm its equivalence. |
| Cross-cultural adaptation | - Translation-backtranslation and piloting.- Piloting of Spanish version in individuals with and without skin disease to assess comprehensibility. | ||||
| Reliability | - Good internal consistency (Cronbach α, 0.7).24 | ||||
| Validity | Content-Assessment of content relevance with patients (n=58)-Evaluation of item clarity, comprehensibility, relevance, and redundancy.Construct- Significantly higher scores for patients with a skin disease than for the general population (P<.01).- Significantly higher scores for patients with inflammatory disease than for those with isolated skin lesions (P<.01).CriterionConvergent- The differences in scores between the Spanish and US versions were significant only for the emotional scale (lower in the Spanish version, P<.05). | ||||
| Skindex-29Jones-Caballero et al.25 (2002): validationProspective observational2C | 318 patients with skin diseases (10% with psoriasis)Mean (SD) age: 36 (15) y- % of male patients: 35% | Tool: Skindex-29 | Reliability | - Good internal consistency (Cronbach α, >0.84).- Good reproducibility (ICC, >0.7). | The Spanish version of Skindex-29 is in general a valid, reliable, and sensitive instrument for measuring the effects of skin disease on HRQoL in Spanish patients. The validation did not include comparisons with other HRQoL questionnaires or clinical variables. The functioning domain showed a considerable floor effect. |
| Validity | Construct-Significantly higher scores for patients with skin disease than for the general population (P<.005).- Significantly higher scores for patients with inflammatory diseases than for those with isolated skin lesions (P<.005). | ||||
| Feasibility | - Adequate ceiling and floor effects. Floor effects >20% were only observed in the functioning domain (28%). A high ceiling effect was observed in <1% of patients in terms of HRQoL impairment.- Low nonresponse rates: 7.5% of individuals left >10% of items blank; nonresponse rate of 0%-1.9% for each item.- Time required for completion: 5min. | ||||
| Sensitivity to change | - Overall effect size (n=40): 0.76. | ||||
| Specific tools for measuring satisfaction with psoriasis treatment | |||||
| CESTEPRibera et al.4 (2011)Prospective observational2C | 423 patients with moderate or severe psoriasisMean (SD) age: 45.9 (13.9) y% of male patients: 61.9% | Disease-related:PASI,Satisfaction with treatment: CESTEP (completion of questionnaire at baseline visit and at 3, 6, 9, and 12 mo) and global assessment of treatment satisfaction using a VAS (0-100)Adherence: Morisky-Green test | Conceptual model | - Clear description of concept being measured.- Identification of domains and selection of items related to satisfaction with psoriasis treatment through a literature review and input from experts and patients. | CESTEP was found to be feasible, valid, and reliable in the target population. |
| Cross-cultural adaptation | - New questionnaire developed for Spanish patients with psoriasis. | ||||
| Reliability | - Good internal consistency (Cronbach α, 0.92).- Adequate reproducibility (ICC, 0.89). | ||||
| Validity | Construct- Factor analysis: a single domain accounted for 54.6% of the variance explained by all the questionnaire items.CriterionConvergent- Weak correlation between CESTEP scores and PASI at baseline visit (r=0.145; P=.003) and strong correlation with VAS (r=−0.806; P=.001). The same tendency was observed during follow-up, with correlation coefficients of 0.38 to 0.33 for PASI and -0.75 to -0.81 for VAS. | ||||
| Feasibility | - Low overall rate of blank responses (1.2%). | ||||
| Sensitivity to change | - Overall effect size: 1.2 at 3 mo, 1.07 at 6 mo, 0.86 at 9 mo, 0.92 at 12 mo. | ||||
Abbreviations: CESTEP, Spanish Satisfaction With Treatment of Psoriasis Questionnaire (SSTPQ); DLQI, Dermatology Life Quality Index; HRQoL, health-related quality of life; ICC, intraclass correlation coefficient; NHP, Nottingham Health Profile; PASI, Psoriasis Area and Severity Index; PDI, Psoriasis Disability Index; VAS, visual analog scale.
Description of Observational HRQoL Studies Conducted in Spain.
| Author (Year), Study Design, and Level of Evidencea | Objective | Description of Sample | HRQoL Variables Analyzed | HRQoL Resultsb | General Conclusions |
|---|---|---|---|---|---|
| López-Estebaranz et al.15 (2016)Cross-sectional2C | To analyze the influence of patient age and a family history of psoriasis on comorbidities and HRQoL in patients with psoriasis. | 1022 patientsType of psoriasis: 100% moderate to severe (PASI >10, BSA >10%, or DLQI >10)% of male patients: 60.3%Age: 18-30 y, 11.4%; 31-60 y, 71.7%; >60 y, 16.9%Family history: 46.8% | Disease-related: PASI, BSAFamily historyTool: DLQI | Mean (SD) DLQI scores18-30 y: 5.1 (5.3)31-60 y: 5.7 (6.5)>60 y 3.8 (5.1)Direct correlation between DLQI scores and PASI (r=0.628, P<.001) and BSA (r=0.609, p<0.001); nonsignificant correlation with number of comorbidities (r=0.016, P=.621).A family history of psoriasis affected patient-perceived HRQoL (OR=1.6; 95% CI, 1.2-2.3; P=.002), regardless of disease severity assessed by PASI (OR=1.1; 95% CI, 1.1-1.2; P=.000). PGA was significantly associated with HRQoL (P<.05).Mean (SD) DLQI scores varied significantly with age (5.1 [5.3] for 18-30 y, 5.7 [6.5] for 31-60 y, and 3.8 (5.1) for >60 y; P=.001). | A family history of psoriasis had a negative impact on patient-perceived HRQoL regardless of disease severity.Younger patients had greater HRQoL impairment. |
| Martínez-Garcia et al. (2014)9Cross-sectional2C | To analyze the influence of psoriasis on anxiety, depression, and QoL of cohabitants of psoriasis patients | 34 patientsType of psoriasis: Severe scalp psoriasis (55%), psoriasis vulgaris with PASI >5 (30%), and genital psoriasis (15%)% of male patients: 50%Mean age: 43 y (range, 19-82 y)Psoriasis duration: 15.6 y49 cohabitants% of male patients: 40.8%Mean age: 40 y | Patient variables: PASICohabitant variables: gender, age, partnership status, relation to patient, educational level, employment situation, level of anxiety and depressionHRQoL tools: DLQI and FDLQI | Mean DLQI score: 12 (range, 1-28).Mean FDLQI score: 8.82 (range, 0-30).Genital involvement had a greater impact on work/studies (mean DLQI score for item 7, 2 vs 0.86; P=.020) and on sex life (mean DLQI score for item 9, 1.8 vs 0.52; P=.008). Scalp involvement had a greater impact on feelings of embarrassment or self-consciousness (mean DLQI score for item 2, 2.33 vs 1.53; P=.03) and on problems caused by treatment (mean DLQI score for item 10, 1.60 vs 0.63; P=.023).Strong association between FDLQI and DLQI scores (r=0.554, P<.001) and PASI (r=0.305, P=. 033).BSA ≥10% associated with higher FDLQI score (11.42 vs 6.32, P=.013). Higher FDLQI score in people living with patients with scalp (11.8 vs 6.76, P=.015) or genital psoriasis (15.40 vs 8.07, P=.031) than with patients with psoriasis in other areas. | Strong association between patient-perceived HRQoL and FDLQI scores, regardless of characteristics of cohabitants. |
| Molina-Leyva et al.16 (2014)Comparative, prospective2C | To explore the prevalence of type D personality in patients with moderate to severe psoriasis and analyze associations with physical and psychological comorbidities and impact on HRQoL | 80 patients from Hospital Universitario GranadaType of psoriasis: 100% moderate to severe psoriasis% of male patients: 50%Mean (SD) age: 43.4 (12.7) yPsoriasis duration: 9 y (range, 3-27 y)Treatments: BIO, 48.7%; CST, 33.7%; TT, 17.5%Comparisons with age- and sex-matched control group | Sociodemographic: age, sexDisease-relatedComorbidities: anxiety/depression, body mass index, hypertension, dyslipidemia, diabetes mellitusOther: type D personality, smokingHRQoL tools: SF-36 and PDI | SF-36 scores (psoriasis vs controls):general health perceptions, 53.0 vs 48.4; physical functioning, 84.6 vs 97.4; physical role functioning, 74.7 vs 90.0; mental health, 61.0 vs 72.0; vitality, 55.1 vs 64.2; bodily pain, 65.3 vs 79.2; social role functioning, 79.3 vs 87.0 (P≤.01); emotional role functioning, 80.8 vs 82.1 (P=.8).PDI scores:daily activities, 21.2; work/studies, 13.3; personal relations, 12.2; leisure, 13.0; treatment, 6.6According to the PDI, patients with psoriasis and type D personality experienced greater impairment in relation to work/studies (P=.02) and personal relations (P=.01) and also had a worse perception of treatment (P=.02).Patients with psoriasis had a 2.1-fold increased risk of developing type D personality.Patients with psoriasis and type D personality were 3.2 times more likely than individuals with type D personality but without psoriasis of developing anxiety. | Patients with psoriasis have worse HRQoL than controls.Type D personality was associated with worse general and sexual impairment and worse HRQoL. |
| Fernández-Torres et al.17 (2014)Cross-sectional2C | To establish the association between characteristics of psoriasis (severity, psoriatic arthritis, treatment) and comorbidities and HRQoL and to identify factors with a greater impact on HRQoL | 395 patients from Hospital Universitario A CoruñaType of psoriasis: plaque% of male patients: 59.7%Mean (SD) age: 50.79 (15.10) yMean (SD) psoriasis duration: 19.53 (13.43) yTreatments: BIO, 16.7%; CST, 30.1%; TT, 51.4% | Sociodemographic: age, sexDisease-related: psoriasis duration, PASIComorbidities: psoriatic arthritisTreatment: BIO vs CST; BIO vs TTHRQoL tool: DLQI | Mean (SD) DLQI score: 4.17 (4.51)Variables associated with worse HRQoL were young age, female sex, shorter psoriasis duration, and higher BSA and PASI scores (P=.000). Patients with longer-duration psoriasis and longer treatment times experienced less HRQoL impairment (OR=0.96; 95% CI, 0.94-0.99; P=.004). A higher comorbidity index was associated with better HRQoL (P=.005).Worse HRQoL in patients being treated with CST or TT compared with BIO (respective mean [SD] scores: 4.36 [4.71], 4.59 [4.57], and 2.91 [3.53] [P=.012]). Psoriatic arthritis was not associated with HRQoL (P=.890).Factors associated with HRQoL impairment in the multivariate analysis were female sex (P=.002), shorter psoriasis duration (P=.004), and treatment type (P=.053). | The main determinants of worse HRQoL were female sex, longer duration of psoriasis, and TT.Disease severity (PASI, BSA) was not associated with HRQoL. |
| Sánchez-Carazo et al.12 (2014)Cross-sectional2C | To determine the association between HRQoL and comorbidities in patients with moderate to severe psoriasis | 1022 patientsType of psoriasis: 89.1% plaque psoriasis, 61% moderate psoriasis, 39% severe psoriasis, 37.3% active disease% of male patients: 60.3%Mean (SD) age: 46.3 (13.8) yPsoriasis duration: 20 y (range, 11.4-29.8 y)Treatments: BIO, 62.8%; CST, 42.6%; PHOTO, 19.0%; TT, 62.8% | Sociodemographic: sexDisease-related: PASI, BSAComorbidities: dyslipidemia, psoriatic arthritis, hypertension, obesity, anxiety, tuberculosis, sleep disorders, depression, diabetes mellitus, cardiovascular diseaseHRQoL tools: SF-36 and DLQI | SF-36PCS (total): 49.7PCS: active psoriasis=48.5 vs nonactive psoriasis=50.4 (P<.001).MCS: 46.2MCS: active psoriasis=43.2 vs nonactive psoriasis=48.0 (P<.001)Lower PCS (worse HRQoL) in patients with psoriasis and psoriatic arthritis, high blood pressure, diabetes mellitus, sleep disorders, or obesity (P<.05). Lower MCS in women and in patients with depression and anxiety (P<.05).Negative correlation between psoriasis severity and PCS (PASI, r=−0.160, P=.0; BSA, r=−0.173, P=.0) and MCS (PASI, r=−0.227, P=.0; BSA, r=−0.214, P=.0).DLQI scoreOverall: 5.2Active psoriasis=9.3 vs nonactive psoriasis=3.0 (P<.001).Patients with psoriasis and anxiety had worse HRQoL according to the DLQI (P<.05).Positive correlation between DLQI and PASI (r=0.628, P=.0) and BSA (r=0.609, P=.0). | Regardless of sex, patients with severe comorbidities, such as psoriatic arthritis, hypertension, and obesity had greater HRQoL impairment, particularly in the physical component.Women had greater impairment in the mental component than men. |
| Daudén et al.2 (2013a)Prospective2C | To evaluate the impact of psoriasis on HRQoL via different evaluation questionnaires | 304 patientsType of psoriasis: 100% plaque psoriasis, 60% active psoriasis, and 40% stable psoriasis% of male patients: 56.3%Mean (SD) age: 45.3 (14.5) yMean (SD) psoriasis duration: 18.3 (12.2) yTreatments: BIO, 38.2%; CST, 51%; TT, 44.1% | Disease-related: psoriasis activity and locationHRQoL tools: DLQI and PDI | DLQI scoresActive psoriasis=7.03 (V1) vs 3.5 (eV); stable psoriasis=2.59 (V1) vs 2.06 (eV)PDI scoresActive psoriasis=8.25 (V1) vs 4.2 (eV); stable psoriasis=3.61 (V1) vs 5.52 (eV)PSO-LIFEActive psoriasis=57.4 (V1) vs 72.2 (eV); stable psoriasis=76.4 (V1) vs 82.3 (eV)PSO-LIFE scores varied according to location of lesions, with greater impairment in patients with lesions in visible areas (head or upper extremities) (mean [SD] score, 63 [22]) compared with less visible areas (trunk and lower extremities) (mean [SD] score, 74 [23.9]) and no lesions at baseline visit (mean [SD] score, 78.5 [21.6]) (P<.01).Moderate correlation between PSO-LIFE scores and PASI (r=−0.4). Correlation between PASI and DLQI (r=0.5) and PDI (0.4) scores.Greater effect size for PSO-LIFE than other HRQoL questionnaires. | Patients with active psoriasis have worse HRQoL.The differences in questionnaire scores between patients with active and stable psoriasis were greater for PSO-LIFE, which had greater discriminatory capacity.Improvement in HRQoL between V1 and eV associated with treatment-related improvement in disease. The PSO-LIFE showed greater sensitivity to change than the other questionnaires.Lesion site and PASI scores were significantly correlated with PSO-LIFE scores. |
| Daudén et al.13 (2013b)Prospective2C | To determine HRQoL in patients with moderate to severe psoriasis | 1217 patientsType of psoriasis: moderate to severe (PASI ≥10, PGA ≥5, BSA ≥10%)% of male patients: 60.8%Mean (SD) age: 45.11 (13.92) yTreatments: BIO, 36.1%; CST, 27.6%; TT, 53.6%; PHOTO, 14.1%; other, 17.5% | Sociodemographic: sex, age, weight, smoking status, educational level, employment situationDisease-related: age at onset, number of flares, PASI, comorbidities, treatment, affected areaHRQoL tools: SF-36, EQ-5D, DLQI, and PDI | SF-36Mean (SD) PCS V1=49.43 (8.83) vs V2=50.81 (8.34)Mean (SD) MCS V1=45.35 (11.96) vs V2=48.07Domains with highest scores at V1: physical functioning (83.67 [21.44]), emotional role functioning (81.33 [23.63]), and physical role functioning (78.02 [25.97]); domains with greatest improvement from V1 to V2: social role functioning (from 76.14 [25.82] to 83.80 [22.09]), physical functioning (from 78.02 [25.97] to 83.58 [22.48]), and bodily pain (from 67.08 [29.35] to 73.56 [27.96]) (P<.001).EQ-5D (VAS)Mean (SD) score V1=64.41 (18.0) vs V2=72.44 (17.88).EQ-5D domains with lowest scores at V1: pain (1.57 [0.58]) and anxiety/depression (1.42 [0.56]); domains with greatest improvement from V1 to V2: pain (1.48 [0.60]) and anxiety/depression 1.35 [0.55])DLQIMean (SD) score V1=8.97 (7.28) vs V2=4.76 (5.72) (P<.001)PDIMean (SD) score V1=9.24 (8.76) vs V2=4.88 (6.66) (P<.001)PASI (b=0.405; P<.001) and sex (b=0.075; P=.048) were significant determinants of HRQoL. | Disease severity was the main factor associated with HRQoL in patients with psoriasis.In all cases, HRQoL improved between V1 and V2. |
| Fernández-Torres et al.10 (2012)Prospective2C | To compare clinical characteristics, prevalence of comorbidities, and HRQoL between patients with psoriasis aged >65 and younger | 371 patientsType of psoriasis: 76% plaque psoriasis% of male patients: 58.8%Mean (SD) age: 50 (14.5) y; 18.9% >65 yPsoriasis duration: 19.3 (13.22) yTreatments: BIO, 18.0%; CST, 32.1%; TT, 47.9%; CST+BIO, 2% | Sociodemographic: ageTreatmentHRQoL tool: DLQI | Mean (SD) DLQI score<65 y=5.49 (6.0) vs> 65 y=3.89 (5.03), (P=.012).Patients treated with CST and BIO had better HRQoL (P=.012). | Patients aged <65 y had worse HRQoL than those aged >65 y.Itching and embarrassment were the most common complaints in both groups.HRQoL was affected by type of treatment. |
| Hernánz et al.14 (2012)Cross-sectional2C | To describe clinical characteristics and treatment profile of patients with moderate to severe psoriasis in Spain and to assess impact on HRQoL | 442 patientsType of psoriasis: 76.2% moderate psoriasis, 23.8% severe psoriasis% of male patients: 62.2%Mean (SD) age: 46.7 (13.9) yPsoriasis duration: 13.1 (11.0) yTreatments: BIO, 57.5%; CST, 32.6%; PHOTO, 11.0%; TT, 27.2%; other, 10.3% | Sociodemographic: ageDisease-related: PASI, psoriasis duration, disease severity and locationComorbidities: psychiatric disordersHRQoL tool: Mean (SD) DLQI score | Mean (SD) DLQI scoresOverall=6.7 (6.6) vs severe psoriasis=9.2 (7.8) and moderate psoriasis=5.9 (6.0) (P<.001).Severe psoriasis was associated with worse DLQI scores in all domains (P<.01 for personal relations and P<.001 for others).60% of patients with severe psoriasis had poor or unsatisfactory HRQoL vs 39.8% of those with moderate psoriasis (P>.001).Patients with better HRQoL were older than those with worse HRQoL (48.0 [13.9] vs 44.9 [13.8], P<.05), regardless of psoriasis severity or psoriasis duration (14.1 [11.8] vs 11.8 [9.7] y, P<.05).Worse HRQoL was associated with greater involvement of the scalp (74.1% vs 48.5%, P<.001), nails (48.7% vs 34.0%, P<.001), genitals (24.9% vs 11.9%, P<.001), and flexural sites (25.9% vs 14.9%, P<.01) and with a greater prevalence of psychiatric disease (15.7% vs 4.5%, P<.001).Factors associated with HRQoL: patient age was a protective factor (OR=0.973; 95% CI, 0.957-0.989); worse HRQoL was associated with scalp involvement (OR=2.260; 95% CI, 1.401-3.645), psychiatric comorbidity (OR=5.105; 95% CI, 2.177-11.972), and PASI (OR=1.067; 95% CI, 1.037-1.098). | 60% of patients with severe psoriasis reported poor or unsatisfactory HRQoL compared with just 39.8% of those with moderate psoriasis (P>.001).Age is a protective factor in terms of the impact of psoriasis on HRQoL while scalp, genital, nail, and flexural involvement, together with psychiatric disease and higher PASI scores were associated with a greater risk of worse HRQoL. |
| Fernández-Peñas et al.18 (2011)Prospective2C | To compare characteristics of different HRQoL tools in patients with different degrees of psoriasis severity | 379 patientsType of psoriasis: 40% severe (PASI ≥12), 32% moderate (PASI 7-12), 24% mild (PASI >7), 4% unknown (unknown PASI) | HRQoL tools: SF-36, DLQI, PDI, and Skindex-29 | Skindex-29 (379 patients), DLQI (144 patients), PDI (133 patients), SF-36 (100 patients).The DLQI, PDI, and Skindex-29 did not detect differences in HRQoL according to sex or age. The SF-36 showed better HRQoL in women than men and the bodily pain domain was correlated with age.Skindex-29 showed a weak to moderate yet significant correlation (symptoms domain r <0.35) (P<.01) with PASI, as did the PDI (r=0.19, P<.05).Substantial floor effect in most (5/6) DLQI domains: daily activities (29%), leisure (36%), personal relations (51%), work and studies (50%), and treatment (31%). The same was observed for PDI (4/5) and SF-36 (5/8) domains. Small floor or ceiling effect (<5%) for Skindex-29 domains. | Most PDI, DLQI, and SF-36 domains have a substantial floor effect, indicating low sensitivity to change from moderate to severe.Skindex-29 showed better sensitivity to clinical severity with a minimal floor effect.Skindex-29 was strongly correlated with the other 3 tools. |
| Melero et al.26 (2011) Retrospective/cross-sectional5 | To determine sociodemographic variables, psoriasis location and type, evaluation time, comorbidities, membership of associations, patient-physician relationship, and knowledge about psoriasis | 200 patientsType of psoriasis: 78% mild to moderate, 53% plaque, 37.5% active disease (flare)% of male patients: 48%Mean age: 46.21 yPsoriasis duration: >10 y in 60.5% of patientsTreatments: not specified | Determinants of HRQoL not analyzedHRQoL tools: SF-36 and Skindex-29 | SF-36Skindex-29(data not reported)84% of participants reported disease worsening with stress and 49.5% indicated that they had experienced an emotional disorder in the past year. | The results showed that emotional factors have a considerable impact on psoriasis symptoms. |
| Ferrándiz-Foraster11 (2007)Cross-sectional2C | To determine the impact of moderate to severe psoriasis on HRQoL measured by the PDI | 3320 patients (90% Spanish, 10% Portuguese)Type of psoriasis: 100% moderate to severe, 80% plaque psoriasis% of male patients: 57%Mean age: 46.74 y (95% CI, 46.19-29)Psoriasis duration: 18 y (95% CI, 17.44-18.46)Treatment: BIO, CST | Sociodemographic: age, sex, personal and family history, number of cigarettesDisease-related: PASI, BSA, overall severity, pruritusDirect costs: visits to dermatologist, days of sick leaveIndirect costs: days of sick leave in past yearWillingness to pay to be free of lesionsTreatment received in last 2 yComorbidities: psoriatic arthritisHRQoL tool: PDI | PDI score=8.93 (95% CI, 7.83-9.21), which represents a disability of 19.9%Floor effect 8.3%-64.5% and ceiling effect 0%-3%.Weak correlation between DLQI and PASI (r=0.275, P<.001) and BSA (r=0.258, P<.001).Worse HRQoL in women (P<.001) and in patients ≤30 y (P<.05).Factors associated with HRQoL (P<.05): female sex, number of cigarettes, PASI, systemic treatment in past 2 y,psoriatic arthritis, monthly expenses due to psoriasis, head lesions, pruritus, no. of visits to dermatologist in past year, days of sick leave in past year, and months of life and percentage of salary willing to give up in order to be lesion-free. | Overall disability level <20%. The correlation between PDI and disease severity (PASI and BSA) was weak but significant. Greater psoriasis involvement was associated with a greater impact on HRQoL. The PDI does not appear to be an ideal tool for assessing HRQoL in this population. |
Abbreviations: BIO, biologic agent; CST, conventional systemic therapy; DLQI, Dermatology Life Quality Index; EQ-5D, EuroQoL-5D; eV, end visit; FDLQI, Family Dermatology Life Quality Index; HRQoL, health-related quality of life; MCS, mental component summary score; PCS, physical component summary score; PDI, Psoriasis Disability Index; PHOTO, phototherapy; SF-36, 36-item Short Form Health Survey; TT, topical treatment; V1, visit 1; V2, visit 2.
Five of the 6 validation studies (Table 1) described the validation of an HRQoL questionnaire for use in Spain (2 of the studies reported on the same questionnaire) and 1 described the validation of a treatment satisfaction questionnaire. Two of the 4 HRQoL questionnaires were designed to assess the impact of generic skin diseases while the other 2 were psoriasis-specific questionnaires developed or validated in Spain. A short description of these questionnaires, together with their characteristics and availability, is provided in Table 3. Their psychometric properties are summarized in Table 4.
Specific PRO Tools Validated or Used in Patients With Psoriasis in Spain.
| PRO | Disease | Tool | Country | General Characteristics | Availability/Access to Spanish Version |
|---|---|---|---|---|---|
| HRQoL | Psoriasis | PSO-LIFE19 | Spain | - Single questionnaire developed to evaluate HRQoL in Spain.- Consists of 20 items rated on a 5-point scale that measure different aspects of HRQoL relevant to patients with psoriasis, including symptoms, impact on emotional well-being, personal relations, activities, and leisure.- Total score ranges from 20 to 100, with higher scores indicating better HRQoL.- To simplify interpretation and validate the questionnaire, the scoring system was converted to a 0-100 scale, with higher scores indicating better HRQoL. | Not available. Request from authors.27 |
| PDI3 | United Kingdom | - Consists of 15 items rated on a scale of 0-3 distributed across 5 domains: daily activities (5 items), work/studies (3 items), personal relationships (2 items), leisure (4 items), and treatment, all relating to the previous 4 weeks.- Total score ranges from 0 to 45, with higher scores indicating better HRQoL.3,19- Interpretation of scores: 0=no disability, 1-4=mild disability, 5-9=moderate disability, 10-18=serious disability, and 19-45=very serious disability.28 | http://sites.cardiff.ac.uk/dermatology/quality-of-life/psoriasis-disability-index-pdi/pdi-different-language-versions/ | ||
| Skin diseases | DLQI21 | United Kingdom | - Consists of 10 items rated on a 4-point scale: symptoms, daily activities, leisure, work/studies, personal relationships, and treatment.- Total score ranges from 0 (minimal impact on HRQoL) to 30 (maximum impact).19- Interpretation of scores (impact of psoriasis on patient's life): 0-1=no impact, 2-5=small impact; 6-10=moderate impact, 11-20=very large impact, and 21-30=extremely large impact.17- Average time required for completion: 2 min.29 | http://sites.cardiff.ac.uk/dermatology/files/2014/07/DLQI-Spanish.pdf | |
| Skindex-2923,25 | United States | - Consists of 3 domains: functioning (12 items), emotions (10 items), and symptoms (7 items). Each item is scored on a 4-point scale.- The score for each domain is obtained by converting the sum of scores to a linear 0-100 scale, where 0 indicates no impact on HRQoL and 100 indicates maximum impact.- Interpretation of scores: mildly impaired HRQoL (cutoff score: 25), moderately impaired HRQoL (cutoff score: 32), severely impaired HRQoL (cutoff score: 44). As a general rule, the cutoff scores for mild, moderate, and severe impairment are rounded off to 20, 30, and 40, respectively, both for the overall and domain scores, with the exception of the symptoms domain (mild=39, moderate=42, and severe=52).30Nijsten et al.31 established a different scoring system, where a score <5 indicates very mild impairment; 6-17 mild impairment; 18-36, moderate impairment; and >37, severe impairment. | Request by e-mail.27 | ||
| Satisfaction | Specific | CESTEP4 | Spain | - Consists of 12 items rated on a scale of 0 (very satisfied) to 5 (very dissatisfied).- Total possible score of 0 (maximum satisfaction) to 48 (maximum dissatisfaction). | Not available. Request from authors.27 |
Abbreviations: CESTEP, Spanish Satisfaction With Treatment of Psoriasis Questionnaire (SSTPQ); DLQI, Dermatology Life Quality Index; HRQoL, health-related quality of life; PDI, Psoriasis Disability Index; PRO, patient-reported outcomes.
Evaluation of Psychometric Properties of Generic Skin Disease and Psoriasis-Specific Patient-Reported Outcome Tools Validated in Spain.
| HRQoL | Satisfaction | ||||
|---|---|---|---|---|---|
| Specific | Specific | ||||
| AttributeaQuestionnaire | PSO-LIFE | PDI | DLQI | Skindex-29 | |
| Conceptual and measurement model | |||||
| Description of concept being measured | + | +++ | +++22 | +++ | +++ |
| Selection and combination of items | ++ | + | ++22 | ++23 | ++ |
| Patient participation in design | +++ | Ø | +++ | ++23 | ++ |
| Cultural adaptation | |||||
| Linguistic equivalence | +++b | + | +++22 | +++23 | +++b |
| Conceptual equivalence | +++ | Ø | ++22 | +++23 | +++ |
| Differences between original and adapted versions | +++ | Ø | Ø | ++23 | +++ |
| Reliability | |||||
| Internal consistency (Cronbach α) | +++ | ++ | + | +++ | +++ |
| Test-retest (internal consistency coefficient) | +++ | Ø | ++ | +++ | ++ |
| Validity | |||||
| Content validity | +++ | Ø | ++ | ++23 | +++ |
| Construct validity | |||||
| Factor analysis | ++ | Ø | Ø | Ø | ++ |
| Known-group/extreme-group method | + | Ø | + | + | Ø |
| Criterion validity | ++ | ++ | + | Ø | ++ |
| Feasibility | |||||
| Viability | |||||
| Floor and ceiling effect | +++ | +18 | + | ++ | Ø |
| Nonresponse rate | +++ | ++ | +++ | +++ | +++ |
| Simplicity | |||||
| Administration mode | ++ | ++ | ++ | ++ | ++ |
| Time required for completion | Ø | Ø | ++ | Ø | Ø |
| Sensitivity to change | |||||
| Effect size test | ++ | +++ | ++ | ++ | +++ |
| Minimal clinically important difference | +++ | Ø | Ø | Ø | Ø |
| Overall score (out of 100) | 78 | 31 | 56 | 61 | 67 |
The 12 observational studies had all used the questionnaires identified (Table 2) to assess HRQoL in patients with psoriasis and, in some cases, other skin diseases. All the studies had used psoriasis-specific questionnaires complemented by generic questionnaires in 5 cases. The Dermatology Life Quality Index (DLQI) was used by 69.2% of the studies, the Psoriasis Disability Index (PDI) and the 36-item Short Form Health Survey (SF-36) by 38.5%, Skindex-29 by 15.4%, and the PSO-LIFE and EuroQoL-5D (EQ-5D) by 7.7%. One study also evaluated the QoL of people living with psoriasis patients using the Family DLQI (FDLQI).9
The results of these studies highlight the negative impact that psoriasis has on both patients and their families (Table 2).
Determinants of HRQoL in Patients With PsoriasisSociodemographic CharacteristicsOn analyzing the impact of psoriasis on HRQoL according to sociodemographic characteristics, we observed a greater impact in women,10–13 young patients,.1014,15 patients with a family history of psoriasis,15 type D (distressed) personality,16 or psoriatic arthritis11,12 or other comorbidities (mainly psychiatric),12,14 and patients who smoked or drank alcohol11,17 (Table 5). A strong correlation was observed between FDLQI and DLQI scores (r=0.554; P<.001), regardless of the characteristics of the cohabitants.9
Determinants of HRQoL in Patients With Psoriasis.
| Sociodemographic Characteristics | Disease-Related Characteristics | Treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (Female) | Age (Young) | Psoriatic Arthritis | Comorbidities (More) | Family History | Type D Personality | Smoking or Alcohol Use | PASI (Greater) | BSA (Greater) | PGA (Greater) | Duration (Greater) | Scalp Involvement | Genital Involvement | BIO | CST | |
| López-Estebaranz et al.15 (2016) | - | x | - | - | - | - | |||||||||
| Molina-Leyva et al.16 (2014) | - | ||||||||||||||
| Fernández-Torres et al.17 (2014) | - | - | x | + | - | - | - | + | +^ | ||||||
| Sánchez-Carazo et al.12 (2014) | - | - | - | - | - | ||||||||||
| Martínez-García et al.9 (2014) | - | - | - | - | |||||||||||
| Daudén et al.13 (2013b) | - | - | |||||||||||||
| Fernández-Torres et al.10 (2012) | - | +¿ | +¿ | ||||||||||||
| Hernánz et al.14 (2012) | - | - | - | - | - | ||||||||||
| Ferrándiz-Foraster et al.11 (2007) | - | - | - | - | - | -* | |||||||||
Abbreviations: BIO, biologic agent; CST, conventional systemic treatment; HRQoL, health-related quality of life; X, no impact on HRQoL.
Symbols: -, negative impact on HRQoL; +, positive impact on HRQoL;
Disease-related factors, such as psoriasis severity, extent of involvement, and time since onset can also affect HRQoL. The clinical variables most strongly associated with HRQoL in Spanish patients with psoriasis were disease severity measured by PASI9,11–15,17 and, to a lesser extent, BSA12,15 (Table 5). Dauden et al.13 showed that the main factors associated with HRQoL were PASI scores (P<.001) and sex (P=.048). Ferrándiz-Foraster et al.,11 in turn, detected weak yet significant correlations (P<.001) between PDI scores and severity measured by PASI and BSA. In a study by Hernanz et al.,14 the strongest predictor of HRQoL was psychiatric comorbidity, with an odds ratio (OR) of 5.105; PASI scores were also predictive, but to a lesser extent (OR=1.067). Fernandez-Peñas et al.18 observed a weak to moderate correlation between PASI and Skindex-29 scores (r<0.35) and this correlation was only significant for some of the questionnaire's domains. The correlation between PASI and HRQoL was nonsignificant for the DLQI (r=0.13) and significant for the PDI (r=0.19).18 Finally, Dauden et al.19 observed a moderate correlation between PASI and PSO-LIFE scores (r=−0.4; P<.01) (Table 1), while López-Estebaranz et al.15 reported that HRQoL measured by the DLQI was strongly correlated with PASI (r=0.628; P<.001) and also associated with PGA (P<.05) (Table 2).
Lesion site is also a significant determinant of impact on HRQoL. Using the PSO-LIFE, Daudén et al.2 found greater HRQoL impairment in patients with lesions in more visible areas (P <.01), and Hernanz et al.14 reported worse DLQI scores in patients with greater involvement of the scalp (74.1% vs 48.5%; P<.001), nails (48.7% vs 34.0%; P<.001), genitals (24.9% vs 11.9%; P<.001), and flexural sites (25.9% vs 14.9%; P<.01).14 Martínez-García et al.9 also found that patients with genital involvement had worse HRQoL in terms of work and studies (P=.020) and sex life (P=.008). The impact on cohabitants was also greater for those living with patients with psoriasis of the scalp (11.8 vs 6.76, P=.015) or genitals (15.40 vs 8.07, P=.031) than those with psoriasis in other areas9 (Table 2).
The studies analyzed also showed that active disease has a greater impact on HRQoL than stable disease.2,12 Other clinical factors, such as psoriasis duration,17 were found to have a positive impact on patient-perceived HRQoL. Finally, patients with longer-duration psoriasis and longer treatment times experienced less impairment (OR=0.96; 95% CI, 0.94-0.99; P=.004).17
TreatmentThree studies assessed the effect of psoriasis treatment on HRQoL. One found better HRQoL in patients being treated with biologic or conventional systemic therapies compared with topical treatments,10 while another found better scores in those receiving biologic drugs compared with topical or conventional systemic therapies.17 The third study found that systemic therapy was a stronger predictor of worse HRQoL than biologic therapy.11
DiscussionAssessment of PROs and HRQoL in particular is gaining importance as a means of assessing population health and the efficacy of health interventions. In psoriasis, HRQoL tools are designed to provide an objective assessment of how psoriasis affects patients’ lives from a holistic perspective.
Measurement of HRQoL in clinical practice requires the use of simple questionnaires that can be completed quickly and that provide information that is both reliable and valid. Creating a new questionnaire is a laborious, costly, time-consuming process, and adapting existing questionnaires to new cultural settings is also a complex process, but a necessary one for cross-cultural studies.
Several Spanish-language questionnaires have already been adapted and validated for use in patients with psoriasis, including generic questionnaires (DLQI21 and Skindex-2925) and questionnaires specifically targeting psoriasis (PDI3). Clinicians and researchers working in Spain can also use the psoriasis-specific PSO-LIFE questionnaire, which was specifically developed and validated for use in this country.19 These questionnaires assess everyday aspects of life that are clearly affected by psoriasis and the information they provide is essential for guiding clinical management.13 Validated questionnaires, however, have certain limitations derived from the validation process and in some cases they may fail to cover aspects of life relevant to Spanish patients, as they were originally designed to be used in other cultural contexts.19
The DLQI21 is the most widely used HRQoL questionnaire because it is both short and simple to use.32 Nonetheless, it has a considerable floor effect (which makes it difficult to detect worsening of perceived HRQoL), does not provide a complete picture of emotional or mental status, and may be insensitive to less evident impairment.32 The Skindex-29 questionnaire25 largely has good measurement properties but it also has a considerable floor effect. The PDI mainly addresses psoriasis symptoms and disability, but some potentially problematic cultural issues have been identified in the Spanish version.13 The PSO-LIFE19 questionnaire is the only questionnaire specifically developed for use in Spanish patients. As it has just 1 scale, its results are reported as a single value, making it easier to score and interpret. It is reliable, valid, and sensitive19 and may therefore be the most suitable instrument for assessing HRQoL in patients with psoriasis.
One observational study conducted by a group of Spanish authors compared the characteristics of 4 HRQoL questionnaires (Skindex-29, DLQI, PDI, and SF-36) in 379 patients with psoriasis. The SF-36 was not sensitive to the effects of psoriasis on HRQoL, and like the DLQI and PDI, it also showed a substantial floor effect. PDI and DLQI scores were correlated with clinical severity in the treatment domains, which measure the impact of treatment rather than severity on HRQoL. Skindex-29 was more responsive to clinical severity and had a minimal floor effect.18
When analyzing HRQoL in patients with psoriasis, it is generally advised to use a generic questionnaire to complement information provided by questionnaires specific to psoriasis.32 The SF-36,33 EQ-5D,34 and Nottingham Health Profile (NHP)35 have all been validated for use in Spanish patients with different skin diseases and subsequently applied to psoriasis. They have all, however, shown little sensitivity to clinical changes in psoriasis.
One notable finding of our literature review is the weak to moderate correlation observed between HRQoL measures and PASI, which is an almost universal tool for assessing clinical severity in psoriasis. We also detected considerable variations in results from one study to the next, with correlation coefficients ranging from r=0.10 (emotional domain) to r=0.35 (symptoms domain) (P<.01)18 in Skindex-29, from r=0.1318 to r=0.628 (P<.001)15 in the DLQI, and from r=0.19 (P<.0.05)18 to r=0.33 (p <0.01)3 in the PDI. The coefficient in the case of PSO-LIFE was r=−0.4.2 These correlations between PASI and HRQoL are generally stronger than previously observed values of between 0.1 and 0.3.36 Nonetheless, they all highlight the importance of assessing both clinical severity and patient-perceived impact on HRQoL. In addition, as these measures correspond to different constructs, they should be presented as independent yet complementary scores. Associations between disease control and HRQoL have also been described for other diseases, such as asthma37,38 and chronic obstructive pulmonary disease.38 In such cases, objective measures such as lung function are not sufficient to explain the impact of disease on patients’ live and must be complemented by HRQoL data.37
ConclusionsThis review shows that PRO tools are not widely used to assess the effects of psoriasis on patients in Spain, even though several studies have detected a negative impact on HRQoL. Possible reasons for this underutilization include time constraints and limited access. As our review shows, however, assessment of PROs and investigation of factors that influence patient perceptions could improve disease management and are a useful adjunct to traditional tools.
FundingThis study was funded by Lilly Spain.
Conflicts of InterestIsabel Belinchón has worked as a consultant for Pfizer-Wyeth, Janssen Pharmaceuticals Inc, MSD, Almirall SA, Lilly, and Leo-Pharma. Luis Lizán and Clara Gabás-Rivera work for an independent research organization and have received fees for their contribution to this project and manuscript. Tatiana Dilla, Teresa Huete, and Silvia Díaz work for Lilly España, which sponsored this study. The authors confirm that the results described in this manuscript, together with their interpretation, are freely expressed opinions and that there were no conflicts of interest in terms of obtaining or reporting these results. The authors report no other conflicts of interest.
Please cite this article as: Lizán L, Gabás-Rivera C, Belinchón I, Dilla T, Huete T, Díaz S. Instrumentos para la valoración de los resultados percibidos por el paciente con psoriasis en España: revisión sistemática de la literatura. 2019;110:561–584.