Interferon beta was approved in Spain in 1995 for the treatment of progressive multiple sclerosis. There are 2 main types, interferon beta-1a and 1b, both of which are sold in a variety of formulations, resulting in a range of responses to the same molecule. Local skin reactions are a common adverse effect, and are usually self-limiting. By contrast, panniculitis and lipoatrophy are very rare, and may necessitate discontinuation of treatment.
We describe the case of a 43-year-old woman who was diagnosed in 2005 with multiple sclerosis, for which she received several disease-modifying treatments. In October 2009, she began treatment with interferon beta-1a (Rebif 44), which was self-administered subcutaneously 3 times a week, with a favorable response. After 18 months of treatment, induration was observed at the injection sites, on the back of the thighs, and on the outer arms. A few weeks later, ulcerated lesions appeared on the front of the thighs and progressively increased in size. The patient reported that the plaques caused severe pain, which made moving and walking difficult. She reported no fever or systemic upset and her neurological status remained unchanged.
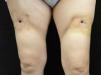
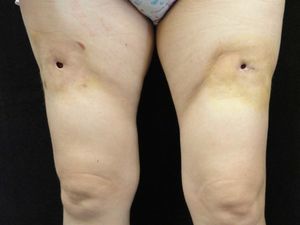
Examination revealed 2 ulcers on the front of the thighs of 1cm diameter. The borders of the ulcers had a scarlike appearance and beneath the ulcers were cavities of about 4cm in diameter. An indurated plaque of about 15cm in diameter, with a slightly erythematous surface, surrounded the ulcer and was intensely painful to touch (Fig. 1). These indurated plaques were also present on the external aspect of both arms, although no ulcers were observed.
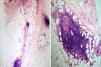
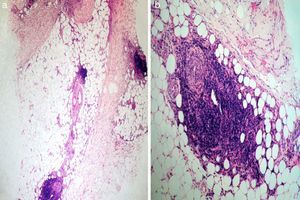
Tissue culture of the ulcers and an incisional biopsy of a plaque from 1 of the thighs were negative. Histopathology revealed a preserved epidermis, sclerosis of the middle and deep dermis, and subcutaneous tissue involvement. At higher magnification we detected an infiltrate that primarily affected the lobule, although septal thickening was also observed (Fig. 2a); this infiltrate was composed mainly of neutrophils and lymphocytes (Fig. 2b). No vasculitis or necrosis was observed. Based on these observations, the patient was diagnosed with mixed panniculitis secondary to interferon beta-1a therapy. Upon consultation with her neurologist, it was decided to discontinue interferon beta-1a treatment, mainly due to functional impairment.
Interferon beta-1a was approved for the treatment of progressive multiple sclerosis in Spain in 1995. Its administration decreases the relapse rate by 30% and the appearance of new lesions on magnetic resonance imaging by 66%. Local skin manifestations such as erythema and pain are well-described frequent adverse effects; they occur in 63% to 85% of patients1 but are usually self-limiting and do not require discontinuation of treatment. However, treatment may need to be discontinued in cases of other more serious reactions such as ulcerations, necrosis,2 sclerosis, and induration at the injection site. Other adverse effects such as sarcoid granulomas, lipoatrofia,3 lupus-like lesions,4 and septal or lobular panniculitis5 are considered very rare. Table 1 summarizes the cases of panniculitis secondary to interferon treatment described to date. Some authors have suggested that this form of panniculitis may be caused by the vascular toxicity of interferon beta.
Patients Described in the Literature Who Developed Panniculitis After Interferon Administration.
| Age, Sex | Location | Onset of Symptoms | Clinical Symptoms | Pathological Findings | |
| Nakamura et al.,52008 | 43, female | Buttocks | 5 y | Erythema, induration, and pain | Perivascular dermatitis and lobular and septal panniculitis with lymphocytic infiltrate |
| Ziegler et al.,61998 | 41, female | Legs | 8 wk | Erythema, induration, and pain | Perivascular infiltrate and lobular panniculitis with lymphocytic infiltrate |
| Heinzerling et al.,72002 | 44, female | Arms, legs and abdomen | 4 y | Painful induration | Septal panniculitis with predominantly lymphocytic infiltrate |
| O'Sullivan et al.,82006 | 37, male | Left leg | 2 y, 3 mo | Erythema and pain | Septal panniculitis with inflammatory infiltrate |
| Ball et al.,92009 | 46, female | Not recorded | 1 y, 10 mo | Pain and induration | Lobular panniculitis with neutrophilic infiltrate |
| Ball et al.,92009 | 45, female | Not recorded | 6 y, 1 mo | Cellulitis | Lipomembranous panniculitis |
| Poulin et al.,102009 | 43, female | Abdomen | 9 y | Erythema, pain, and fever | Septal panniculitis with predominantly lymphocytic infiltrate |
| Present case | 43, female | Legs | 19 mo | Pain, erythema, induration, and functional impairment | Lobular and septal panniculitis with mixed inflammatory infiltrate (lymphocytes and neutrophils) |
In 2009, Ball and coworkers9 reported that interferon beta-induced panniculitis can mimic pancreatic panniculitis; differential diagnosis in such cases may be facilitated by lipase and amylase tests.
Two theories have been proposed regarding the pathophysiology of panniculitis secondary to interferon beta treatment. The first proposes that the stress associated with self-injection plays a key role in triggering this condition. The second theory attributes greater importance to the immunological effects of interferon beta. According to the latter theory, the resulting symptoms reflect activation of the immune system and may be an indication of treatment effectiveness.9
The management of patients with panniculitis secondary to interferon beta treatment remains unsatisfactory. It is essential that patients are shown how to correctly self-administer the injection, which should be subcutaneous and never intradermal, and to rotate the injection sites daily. Application of these measures leads to improvements in the majority of lesions,5 although in some cases suspension of interferon beta treatment may be necessary, a decision that should be made in collaboration with the other specialists treating the patient.
Please cite this article as: Cuesta L, et al. Paniculitis mixta secundaria al uso de interferón β 1A en una paciente con esclerosis múltiple. Actas Dermosifiliogr. 2013;104:257–9.