Lentigo maligna is the most common form of in situ melanoma. It is most often found on the head and neck, and its clinical and dermoscopic features in this location have been extensively described in the literature. We present a series of 14 patients diagnosed with extrafacial lentigo maligna and lentigo maligna melanoma at Hospital General de Valencia and Hospital de Manacor in Spain, and describe the clinical, dermoscopic, and histologic features observed. Most of the melanomas were located on the upper limbs; the next most common locations were the trunk and the lower limbs. The dermoscopic patterns were consistent with facial lentigo maligna and superficial spreading melanoma. Extrafacial lentigo maligna is uncommon. It has similar clinical and histologic features to facial lentigo, but dermoscopy may show a mix of patterns typically seen in lentigo maligna and superficial spreading melanoma. This difference in dermoscopic features is essentially due to anatomical differences between skin on the face and on other parts of the body.
El lentigo maligno es actualmente la variante más frecuente de melanoma in situ. Se localiza principalmente en la cabeza y el cuello, donde sus características clínicas y dermatoscópicas están descritas ampliamente en la literatura. Presentamos una serie de 14 pacientes diagnosticados de lentigo maligno y lentigo maligno melanoma extrafacial en el Hospital General de Valencia y Hospital de Manacor, describiendo los hallazgos clínicos, dermatoscópicos e histológicos de los mismos. La localización más frecuentemente encontrada fue las extremidades superiores, seguida del tronco y las extremidades inferiores. En la dermatoscopia se hallaron patrones compatibles con lentigo maligno facial y melanoma de extensión superficial. La localización extracefálica de los lentigos malignos es poco frecuente. Clínica e histológicamente muestra características semejantes al de localización facial, aunque en la dermatoscopia podemos encontrar una mezcla de patrones de lentigo maligno y melanoma de extensión superficial, debido principalmente a las diferencias entre las características anatómicas de la piel facial y la extrafacial.
The term lentigo maligna (LM) was first described by Hutchinson in 1890.1 It is used to describe melanoma in situ that develops on chronically sun-exposed skin in elderly individuals.2 Approximately 86% of all cases occur in the head and neck region, which is by far the most common site affected.3
The term lentigo maligna melanoma (LMM) is used to describe the invasive form of LM. In Spain LMM accounts for 8% of all invasive melanomas excised annually.4
The majority of studies of LM to date have analyzed lesions on the head and neck, with few series analyzing extrafacial locations.3,5,6
Material and MethodsWe undertook a descriptive study of the clinical, dermoscopic, and histologic characteristics of extrafacial LM/LMM diagnosed at 2 Spanish hospitals: Hospital General Universitario de Valencia (HGU) and Hospital de Manacor (HMAN).
The main inclusion criterion was that the specimens had been examined by the same dermatopathologist. At the time of the study, the database at HGU contained information on 296 LM and LMM cases diagnosed since 1990, and 44 of these were extrafacial. Following histologic confirmation of extrafacial LM and LMM, only those tumors with a clinical and a dermoscopic image of the lesion were selected for inclusion in the study (n = 11). At HDM, only lesions diagnosed in 2014 and 2015 and that met the study criteria were included.
For all specimens, sections measuring 4 μm were cut and stained with hematoxylin and eosin. LM was defined as a melanoma in situ formed by a proliferation of atypical, predominantly single, melanocytes along the basal layer of the epidermis and adnexal structures with poorly defined borders, accompanied by epidermal atrophy and solar elastosis.2,3 These melanocytes occasionally form small nests at the dermal-epidermal junction and are typically surrounded by a light halo caused by a retraction artifact. There may also be multinucleated giant cells in the epidermis, and upward (pagetoid) spread of melanocytes is not a prominent finding. Melanophages are observed in the papillary dermis. LMM was defined using the same criteria as above in addition to dermal invasion by neoplastic cells. Breslow thickness was measured in all invasive lesions.
Superficial spreading melanoma (SSM) differs from LM in that it does not generally show signs of significant sun damage, the epidermis is acanthotic, and there are solitary or nested epithelioid cells with abundant cytoplasm and clumps of melanin distributed throughout the epidermis. Mitotic activity is also observed, together with pagetoid spread (a common finding) and regression phenomena with an accompanying inflammatory infiltrate. The above criteria were used to differentiate between LM/LMM and SSM.
ResultsWe studied 8 women and 6 men with a mean age of 75 years (median, 78 years) (Table 1).
Clinical Characteristics of Extrafacial Lentigo Maligna and Tumor Stage in 14 Patients.
| Hospital | Sex | Age, y | Location | Max Diam, mm | Breslow Thickness, mm | Stage | |
|---|---|---|---|---|---|---|---|
| 1 | HGV | Male | 85 | Scapula | 15 | 0.2 | T1aN0M0 |
| 2 | HGV | Female | 88 | Hand | 30 | 0.38 | T1aN0M0 |
| 3 | HGV | Male | 82 | Scapula | 10 | Tis | TisN0M0 |
| 4 | HGV | Female | 73 | Upper arm | 6 | Tis | TisN0M0 |
| 5 | HGV | Male | 78 | Shoulder | 20 | 0.3 | T1aN0M0 |
| 6 | HGV | Female | 73 | Leg | 15 | Tis | TisN0M0 |
| 7 | HGV | Female | 78 | Forearm | 21 | Tis | TisN0M0 |
| 8 | HGV | Female | 57 | Leg | 8 | Tis | TisN0M0 |
| 9 | HGV | Female | 84 | Upper arm | 17 | Tis | TisN0M0 |
| 10 | HGV | Female | 83 | Forearm | 6 | Tis | TisN0M0 |
| 11 | HGV | Male | 49 | Shoulder | 35 | 0.2 | T1aN0M0 |
| 12 | HMAN | Male | 63 | Scapula | 13 | Tis | TisN0M0 |
| 13 | HMAN | Male | 72 | Forearm | 10 | 0,23 | T1aN0M0 |
| 14 | HMAN | Female | 85 | Pretibial region | 15 | Tis | TisN0M0 |
Abbreviations: Max Diam, maximum diameter; HGV: Hospital General Universitario de Valencia; HMAN, Hospital de Manacor; Tis, melanoma in situ.
Eight lesions were located on the upper limbs, 3 on the trunk (the scapula), and 3 on the lower limbs. The mean maximum diameter was 15.78mm. Clinically, most of the lesions were described as slow-growing, asymmetric macules with poorly defined borders, heterogeneous pigmentation, and colors ranging from light to dark brown with white-grayish areas of regression.
Dermoscopic examination of the lesions showed characteristic findings of both LM and SSM. We searched for the presence of dermoscopic features described for facial LM, namely rhomboidal structures (present in 9/14 lesions), annular-granular pigmentation (5/14), asymmetric perifollicular pigmentation (3/14), obliteration of follicular openings (3/14), increased vascular network (2/14), red rhomboidal structures (2/14), and isobar structures, and darkening at dermoscopic examination (0/14). We also evaluated dermoscopic signs associated with SSM, i.e., regression (12/14), an atypical network (10/14), irregular dots or globules (10/14), pseudopods (4/14), a blue-white veil (4/14), and black lamella (0/14). These findings are shown in Table 2.
Dermoscopic Features of Extrafacial Lentigo Maligna in 14 Patients.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dermoscopic findings described for lentigo maligna | APFO (21.4%) | ||||||||||||||
| AG pattern (35.7%) | |||||||||||||||
| Rhom. structures (64%) | |||||||||||||||
| Obliterated FOs (21.4%) | |||||||||||||||
| Isobar structures (0%) | |||||||||||||||
| Increased VN (14.7%) | |||||||||||||||
| Red rhom. structures (7.14%) | |||||||||||||||
| Dermoscopic darkening (0%) | |||||||||||||||
| Dermoscopic findings described for superficial spreading melanoma | Atypical network (71.4%) | ||||||||||||||
| Pseudopods (28.57%) | |||||||||||||||
| Irregular dots/globules (71.4%) | |||||||||||||||
| Blue-white veil (28.57%) | |||||||||||||||
| Black lamella (0%) | |||||||||||||||
| Regression (85.7%) |
Abbreviations: AG, annular-granular; APFOs, asymmetric pigmented follicular openings; FOs, follicular openings; Rhom., rhomboidal; VN, vascular network.
We recorded 17 histologic features, shown in Table 3. Mean Breslow thickness for the 5 invasive lesions was 0.26mm.
Histologic Features of Extrafacial Lentigo Maligna in 14 Patients.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal atrophy | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Variable | Yes |
| Solar elastosis | Marked | Nodular | Marked | Mild | Mild | Mild | Mild | Mild | Mild | Marked | Mild | Marked | Mild | Thin |
| Proliferation of melanocytes in basal layer | 3:1 | 1:1 | 2:1 | 1:2 | 2:1 | 2:1 | 3:1 | 2:1 | 1:1 | 1:1 | 1:1 | 3:1 | 2:1 | 2:1 |
| Involvement of adnexal structures | Folliclular | Eccrine | No | No | Folliclular | Eccrine | Eccrine/follicular | Eccrine | Eccrine | Eccrine/follicular | No | No | Eccrine | Eccrine/follicular |
| Epithelioid melanocytes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Spindle melanocytes | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No |
| Light halo around melanocytes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Dendritic melanocytes | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Melanophages in papillary dermis | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | |
| Small nests | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Pagetoid spread | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Focal |
| Apoptotic cells | No | Yes | No | No | Isolated | No | Yes | No | Isolated | No | Isolated | No | Yes | No |
| Intraepidermal mitosis | Isolated | No | No | No | No | Isolated | No | No | Isolated | No | No | No | Yes | No |
| Giant cells | Yes | No | No | No | Yes | No | No | No | No | No | Yes | No | No | No |
| Inflammatory or lymphocytic infiltrate | Yes | No | Mild | Mild | Moderate | Mild | Mild | Mild | Mild | No | Mild | Mild | Mild | Mild |
| Fibrosis | No | No | No | No | No | Yes | Yes | NA | No | No | Yes | Yes | No | No |
| Vascular proliferation | Yes | No | No | No | No | +++ | ++ | NA | No | No | ++ | No | ++++ | No |
Abbreviation: NA, not assessed.
LM has low malignant potential and is characterized by slow progression to the invasive form LMM. Invasive lesions have a similar prognosis to SSM.
The clinical and dermoscopic appearance of lesions are determined by the characteristics of the sun-damaged skin in which they arise (epidermal atrophy and effacement of rete ridges).
Clinically, LM presents as a slow-growing pigmented macule in chronically sun-exposed areas of the skin (Fig. 1). It is characterized by asymmetric pigmentation and morphology, and has poorly defined borders.2 Extrafacial LM was first described by Hutchinson,1 but these lesions account for less than 20% of all LM cases.2,3
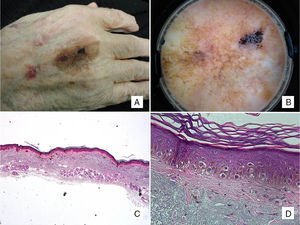
Patient #2: An 88-year-old man with a pigmented macule present on his hand for years. A, Pigmented macule with asymmetric morphology and pigmentation. B, Dermoscopic image showing annular-granular pigmentation at one of the borders, together with rhomboidal structures and areas of regression. C, Histologic image of excised lesion showing proliferation of single melanocytes surrounded by a light halo in sun-damaged skin (marked solar elastosis) in addition to epidermal atrophy (hematoxylin-eosin, original magnification ×20). D, A higher-magnification view shows a proliferation of epithelioid cells with a pagetoid distribution and subepidermal fibrosis (hematoxylin-eosin, original magnification ×200).
Dermoscopic features of facial LM have been extensively described and include dark and red rhomboidal structures, asymmetric perifollicular pigmentation, obliteration of follicular openings, annular-granular pigmentation, isobar structures, and an increased vascular network.6–9 These features are largely determined by the structural characteristics of the skin on the face (effaced rete ridges and prominent adnexal structures). These distinctive anatomic characteristics must therefore be borne in mind when interpreting lentigo-like dermoscopic findings in locations other than the face (Fig. 2).
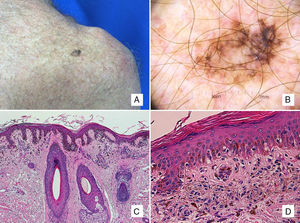
Patient #4: A 63-year-old man with a pigmented lesion on the scapula. A, A 13-mm pigmented macule on the left scapula. B, Dermoscopic image showing asymmetric follicular pigmentation, rhomboidal structures, and some obliterated follicular openings. Note also the features typically associated with superficial spreading melanoma, such as the atypical network, regression areas, and a blue-white veil. C, Proliferation of solitary and small nests of epithelioid melanocytes in the epidermis, with an accompanying inflammatory infiltrate, involvement of the adnexal epithelium, and solar elastosis (hematoxylin-eosin, original magnification ×100). D, A higher-magnification view shows melanophages in the papillary dermis (hematoxylin-eosin, original magnification ×200).
In the largest series of LM/LMM published to date, Cox et al.3 described a prevalence of 17.5% for extrafacial lesions; most of the patients were women (49 vs 22 men) and the mean age was 63 years, which is somewhat lower than that in our series (75 years). Similarly to in our patients, the lesions were located predominantly on the trunk (68%) in men and on the limbs (80%) in women. Cox et al. did not report any clinical or dermoscopic features.
Some years later, Lau et al.5 described the dermoscopic findings of LM on the upper limbs of 3 women. They highlighted the presence of structures associated with both SSM (network, dots/globules, pseudopods) and LM (rhomboidal structures and perifollicular pigmentation) and concluded that these 2 patterns coexist and that the presence of rhomboidal structures and asymmetric perifollicular pigmentation would point to a diagnosis of LM.
In 2014, Keir6 reported on a series of 20 patients with extrafacial LM in which 90% of lesions had a poorly defined border and a lentigo-like pattern, defined as any combination of fine reticular lines, fingerprint-like structures, and light brown areas. Ten (71%) of the 14 lesions in our series had these features, supporting their potential diagnostic value.
The histologic differences between LMM and SSM were described by Clark et al. in 1969.10 For SSM, the authors described nests of cells with a more monomorphous appearance than in LMM and a greater pagetoid distribution of melanocytes. For LMM, they described a predominance of epidermal atrophy, with proliferation of single melanocytes and a greater degree of cellular pleomorphism, in addition to involvement of adnexal structures and less frequent melanocytic nest formation. In our series, isolated nests were observed in 13 of the 14 cases, while focal pagetoid spread of melanocytes was observed in 11 cases. Cytologically, our findings were consistent with the descriptions by Clark et al., with marked cellular pleomorphism and more epithelioid than spindle cells.
Histologic findings did not differ to those described for facial LM.
ConclusionsLM progresses very slowly and may therefore be present for years. This slow-growing melanoma, however, has excellent prognosis. Most lesions are in situ and if they acquire invasive potential, they generally have a low Breslow thickness, although with time they can reach the same depth as any melanoma. Dermoscopic features include a mix of patterns seen in facial LM and SSM, although typical features of SSM are dominant in larger lesions. The literature to date contains few reports of extrafacial LM/LMM, and more studies are needed to identify specific criteria to improve the accuracy of clinical diagnosis, as based on the data available so far, extrafacial LM has overlapping dermoscopic features with SSM.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martínez-Leboráns L, Garcías-Ladaria J, Oliver-Martínez V, Miquel VA. Lentigo maligno extrafacial. Serie de 14 casos y revisión de la literatura. Actas Dermosifiliogr. 2016;107:e57–e63.