BRAF inhibitors with epidermal growth factor receptor (EGFR) inhibitors are increasingly being deployed as a second-line therapy of BRAF V600E-mutant metastatic colorectal cancer (mCRC).1 A well-reported side effect of BRAF inhibitors monotherapy is the development of melanocytic proliferations and cutaneous squamoproliferative lesions, such as squamous cell carcinomas, keratoacanthomas and verrucous papillomas.2,3 Eruptive nevi have been reported as a side effect of combining encorafenib and cetuximab.4,5 However, epithelial proliferations have not been documented with this association.
We report a case of eruptive verrucous keratoses and melanocytic nevi following treatment with encorafenib plus cetuximab for mCRC.
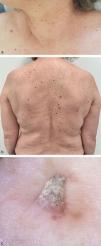
A 60-year-old woman had been treated for BRAF-mutated metastatic colorectal cancer. She had undergone 5 cycles of chemotherapy with folinic acid, fluorouracil, and oxaliplatin (FOLFOX) plus bevacizumab, followed by left hemicolectomy and liver metastasectomy. Due to progressive disease, second-line combination therapy with encorafenib and cetuximab was started. Two months later, she was referred to our department after growing multiple wart-like lesions and melanocytic nevi on her upper body. Physical examination revealed 5 yellow-white, hyperkeratotic papules measuring 2–6mm in diameter, round to filiform in shape, scattered over the face, neck, and shoulders. Multiple melanocytic nevi were also observed on the back (Fig. 1(a)–(c)). Histopathology showed features of verrucous keratosis without atypical keratinocytes. Two months later, the patient returned for follow-up and exhibited 6 new verrucous hyperkeratotic papules, with similar histopathology. The melanocytic nevi remained stable.
(a) Clinical signs of eruptive BRAF inhibitor–associated verrucous keratosis: yellow-white hyperkeratotic, 2–6mm in size, rounded or filiform papules, scattered around the upper body; (b) Multiple new-onset melanocytic nevi on the back; (c) Dermoscopy of BRAF inhibitor-associated verrucous keratosis: yellow-white hyperkeratotic filiform papule.
BRAF inhibitor-associated verrucous keratosis (BAVK) are benign lesions that occur in up to 60% of patients on BRAF monotherapy and have been associated with an increased risk of squamous cell carcinoma and keratoacanthoma.2,3,6 Although eruptive melanocytic nevi are less common, occurring in 10% of the patients, they have been well-documented in this context.2,3,7 The malignant potential of melanocytic proliferation remains uncertain, although cases of secondary primary melanoma have been reported during BRAF inhibitor monotherapy.2
The use of BRAF inhibitors has expanded beyond melanoma, now serving as a cancer target across various cancer types such as non-small-cell lung cancer, anaplastic thyroid carcinoma and hairy cell leukemia. They have been explored both as monotherapy and in combination with MEK inhibition in BRAF V600-mutant cancers and, recently, with EGFR inhibition in colorectal cancer. Despite promising results in monotherapy trials, the development of resistance and adverse drug effects hinders their widespread use.3,8
The inhibition of BRAF paradoxically activates the MAPK pathway in wild-type-BRAF cells, leading to keratinocyte and melanocytic proliferation in normal skin.3,8
Clinical trials, mainly on metastatic melanoma, showed that the addiction of MEK inhibitors demonstrates a protective effect against the onset of verrucous keratosis, resulting in a drop in frequency down to 0–7%.6,8,9 This protective effect extends to melanocytic proliferation, although specific frequency details are currently lacking in the literature.6 Data on the use of these therapies in other malignancies, including colorectal cancer, are currently lacking, despite their widespread use.
Although EGFR inhibitors may similarly promote proliferation through paradoxical activation of the MAPK pathway,10 no cases of cetuximab-induced squamoproliferative or melanocytic lesions have been reported, despite its widespread use in various malignancies. Therefore, in the present case, the eruptive verrucous keratosis and melanocytic nevi would be induced by encorafenib rather than cetuximab.
In conclusion, we report a case of eruptive verrucous keratosis and melanocytic nevi due to encorafenib plus cetuximab therapy. In our case, therapy was continued despite the new BAVK lesions, but due to the risk of association with squamous cell carcinoma and keratoacanthomas, we recommend close follow-up. Encorafenib plus cetuximab, with or without binimetinib, has demonstrated a similar overall survival benefit in previously treated patients with BRAF-mutated mCRC,1 suggesting that the combination without a MEK inhibitor will be used more frequently and may lead to a reemergence of these adverse effects. Awareness of these side effects and a thorough dermatological assessment before and during treatment with BRAF inhibitors is highly recommended.
Conflict of interestThe authors declare that they have no conflict of interest.