Ulcers are a frequent cause of dermatologic consultation, and most correspond to leg ulcers. Major advances in the treatment of ulcers have occurred in recent years as a result of research that has led to new concepts such as the consideration of the chronic ulcer as an inflammatory process involving proinflammatory cytokines and deficits of growth factors. Furthermore, studies into the use of the wet dressing have led to the appearance of a wide variety of new dressings. The aim of this review is to update the reader's knowledge of the treatment of ulcers in general and of leg ulcers in particular, with a detailed description of the new dressings available and of the new therapies for use in refractory cases.
El tratamiento de las úlceras, incluyendo su forma más frecuente, las úlceras en las piernas, es un motivo de consulta frecuente para el dermatólogo. En los últimos años se han producido importantes avances en el campo del tratamiento de las úlceras, como el concepto de la úlcera crónica como un proceso inflamatorio con implicación de citoquinas proinflamatorias y déficits de factores de crecimiento, o la teoría de la cura húmeda que ha originado la aparición de gran variedad de apósitos. La presente revisión intenta actualizar los conocimientos del lector en la cura de las úlceras en general y de las úlceras en las piernas en particular, con especial hincapié en los nuevos apósitos y en las terapias avanzadas para los casos refractarios.
It seems appropriate to begin a review of the diagnosis and treatment of leg ulcers with a definition of what we mean by the term ulcer. In dermatology, an ulcer is a pathologic process that causes loss of skin integrity in at least the dermis. In English, when the ulcer is caused by trauma it is called a wound (herida in Spanish). The term ulceration is preferred for an acute process, while ulcer is used for chronic conditions1 and the term chronic ulcer would be tautological. We should also define what we mean by chronic. Some reviews anchor the concept to a specific period between 4 to 12 weeks, and use the term chronic wound when healing exceeds this time.2 However, the most widely accepted definition is to consider an ulcer to be chronic if it does not heal in the expected time or shows little tendency to heal.3,4 This review will deal specifically with leg ulcers, an area in which in my opinion Spanish dermatologists should play a much more prominent role, as occurs in other countries. We should not forget that dermatologists such as Falanga and Eaglstein have made essential contributions to the field of wound healing and the modern concepts of wound care. Nevertheless, in Spain almost no dermatologists attend conferences on chronic wounds and the pharmaceutical industry has turned its back on the specialty. The result is that Spanish dermatologists have been bystanders to the impressive progress in this field over the last 15 years. Fortunately, I am not alone among Spanish dermatologists in my endeavor to change this situation.5 There have been many studies on the epidemiology of leg ulcers, but very few in the Spanish population. In a meta-analysis of several studies in different countries, it was found that the prevalence in adults ranged from 0.12% to 1.1%.6 The first epidemiological study of leg ulcers in Spain found a prevalence of 0.16%, consistent with the international findings.7 What is rather worrying is that it is thought that only 50% receive appropriate treatment and 25% do not have an etiologic diagnosis.8 This situation creates a burden on health care resources estimated at 7000 million dollars a year in the United States and in Spain generates 1 391 496 consultations in primary health care annually.9
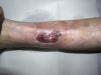
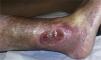
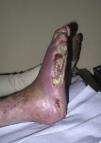
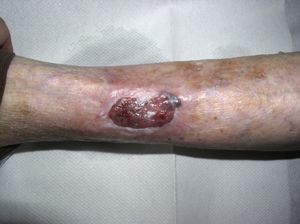
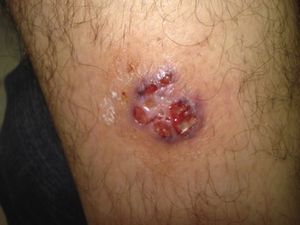
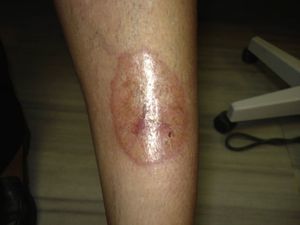
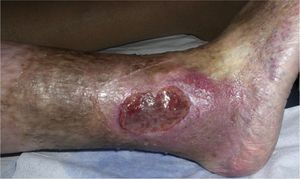
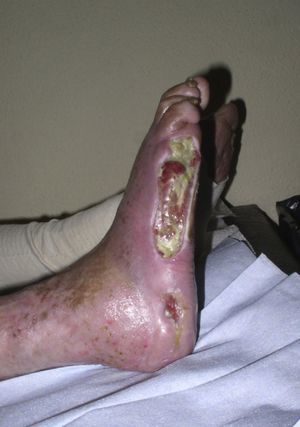
Differential Diagnosis of Leg UlcersDifferential diagnosis of leg ulcers involves consideration of a broad range of conditions (Table 1). We must start from the premise that, no matter how good the wound care is, treatment of the underlying cause is important for healing the ulcer and therefore correct diagnosis is essential. In routine clinical practice, when patients attend their family physician with a leg ulcer, they are usually referred directly to the nursing service without any prior assessment. Most leg ulcers are of vascular origin (85%), including venous ulcers (60%), arterial ulcers, and those that are a combination of both. Neuropathic ulcers account for a further 5% of the total and occur mainly in patients with diabetes. The remaining 10% of these patients have ulcers of essentially dermatologic origin caused by a variety of conditions, including vasculitis, tumor infections (Fig. 1), pyoderma gangrenosum (Fig. 2), and necrobiosis lipoidica (Fig. 3).
Causes of Leg Ulcers.
| Venous Insufficiency |
| Arterial Occlusion |
| ○ Arteriosclerosis |
| ○ Thromboembolism |
| ○ Cholesterol embolism |
| ○ Thromboangiitis obliterans |
| Changes in Microcirculation |
| ○ Raynaud phenomenon and scleroderma |
| ○ Hypertension (Martorell ulcer) |
| ○ Increase in blood viscosity (paraproteinemias, leukemias, etc) |
| Physical or Chemical Lesions |
| ○ Pressure ulcers |
| ○ Trauma, burns |
| ○ Corrosive agents (sclerotherapy) |
| Infections |
| ○ Bacterial: ecthyma, ecthyma gangrenosum, gas gangrene, necrotizing fasciitis, anthrax, carbuncle, syphilis, Buruli ulcer, leprosy, lupus vulgaris tuberculosum, tuberculous carbuncle |
| ○ Viral: chronic herpes, cytomegalovirus-induced ulcers |
| ○ Parasites and fungi: leishmaniasis, Madura foot, sporotrichosis |
| Neuropathic Ulcers |
| ○ Diabetes and other processes with decreased sensitivity (syringomyelia, tabes dorsalis, etc.) |
| Vasculitis |
| ○ Leukocytoclastic vasculitis, polyarteritis nodosa |
| Hematologic Diseases |
| ○ Sickle cell anemia, thrombocythemias, Waldenström macroglobulinemia, myeloma |
| Coagulation Disorders |
| ○ Antiphospholipid syndrome; factor v, xiii, antithrombin iii, protein C or S deficiency; purpura fulminans |
| Cutaneous Tumors |
| ○ Basal cell carcinoma, squamous cell carcinoma, lymphomas, sarcomas |
| Drug-induced Ulcers |
| ○ Hydroxyurea, cytostatic extravasation |
| Cutaneous Diseases |
| ○ Pyoderma gangrenosum, necrobiosis lipoidica, panniculitis, indurated erythema, cutaneous lupus erythematosus |
| Metabolic Diseases |
| ○ Diabetes, prolidase deficiency, calciphylaxis, calcinosis cutis, porphyria cutanea tarda, hyperoxaluria |
| Miscellaneous |
| ○ Chromosome disorders: Klinefelter syndrome (xxy), xyy |
| ○ Rheumatoid arthritis |
The most common difficulty in daily practice is to distinguish between a leg ulcer of venous origin (Fig. 4) and arterial origin (Fig. 5). The considerations presented in Table 2 can be used as a guide.
Differential Diagnosis of Venous and Arterial Ulcers.
| Venous Ulcer | Arterial Ulcer | |
| Site | Supramalleolar region | Dorsum of the foot, toes |
| Appearance | Excavated edgesBleeding bed | Flat edgesFlat bed |
| Perilesional skin | PigmentationDermatitis | Pale skin, coldLoss of skin appendagesUngual dystrophy |
| Pain | LimitedImproves in decubitus position with leg raised or with compression therapy | SubstantialWorsens in decubitus position or when walking |
| ABI | >0.8 | <0.60.8-0.6=mixed |
Abbreviation: ABI, ankle-brachial index.
Irrespective of the cause of the ulcer, a minimal assessment should be undertaken, and this should sometimes be complemented with a more in depth evaluation.
Minimal Assessment of a Patient With Leg UlcerMedical HistoryThe first step should be to collect information on underlying diseases (such as hypertension, diabetes, arteriosclerotic disease, and thrombotic disease) and the use of any drugs that might affect wound healing or cause the ulcer (hydroxyurea, anticoagulant therapy, cytostatic agents, nonsteroidal anti-inflammatory agents, colchicine, and corticosteroids or other immunosuppressive agents). The time course of the ulcer, prior treatments, and previous episodes of ulcer are also of interest. Longstanding ulcers in very elderly patients may be a manifestation of cellular senescence, as discussed later. Pain and its time course can help differentiate between venous and arterial ulcers. Pain derived from venous ulcers worsens in situations in which venous pressure increases, such as when standing, and remits when venous pressure decreases, for example, when walking, resting with the legs raised, and with application of compression therapy. Pain associated with arterial ulcers is experienced at rest or when walking.
Physical ExaminationThe first action should be palpation of the pedal pulses to assess a possible arterial component. We should then pay attention to the state of the surrounding skin for further diagnostic information (presence of varicose veins, lipodermatosclerosis, hyper- or hypopigmentation, signs of vasculitis, signs of necrobiosis lipoidica, etc) and check for complications that might necessitate intervention, such as dermatitis or signs of cellulitis. The examination of the ulcer itself should include the site, depth, state of the borders (macerated, sclerotic, suggestive of carcinoma, blistered, suggestive of pyoderma gangrenosum, etc), presence of nonviable tissue (necrosis), quantity and type of exudate, presence of granulation tissue, and ulcer size.
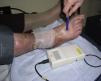
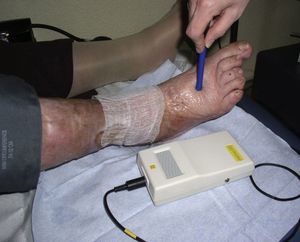
Calculation of the Ankle-Brachial IndexThe ankle-brachial index should be calculated for all patients using a handheld Doppler device (Fig. 6). Without going into details, the technique consists of measuring systolic blood pressure in the pedal or posterior tibial artery and dividing by the pressure in the radial artery. A value close to 1 is considered normal, and we can apply compression therapy if the ulcer is of venous origin. If the value is less than 0.6, the ulcer is considered of arterial origin and compression is contraindicated. If the value is between 0.6 and 0.8, we can use inelastic systems, as discussed later.
Laboratory DeterminationsIn all cases, we should carry out a routine laboratory workup to rule out hypoproteinemia and anemia, conditions which, along with hypovitaminosis C, A, and E, can be associated with delayed wound healing.
Specific AssessmentLaboratory DeterminationsThe laboratory assessments should be guided by the suspected diagnosis. Possible tests include measurement of antinuclear antibodies; rheumatoid factor; antiphospholipid antibodies; syphilis markers; assessment of hematologic disorders; assessment of prothrombotic states due to antithrombin iii, Leiden factor v, and protein S or C deficiency; and karyotyping to rule out xxy or xyy trisomy.
CultureI include culture as a specific evaluation as it should not be performed routinely. Specific indications are presented below. The gold standard is quantitative culture of samples obtained by biopsy or aspiration. This should not only identify the infectious agent but also give the number of colony forming units per gram of tissue. In practice, qualitative cultures are more often used. Samples are taken with a sterile loop. If this method is used, clean the ulcer with saline solution before sampling, avoid taking the sample from necrotic tissue, and obtain at least 2 samples.
BiopsyBiopsy should be done when a tumor or some other condition that can be diagnosed by histologic examination is suspected. Examples of such conditions include vasculitis, pyoderma gangrenosum, and necrobiosis lipoidica. Biopsy should also be performed when an ulcer does not respond satisfactorily to appropriate treatment within a reasonable interval of, for example, 3 months. In such cases, biopsy can rule out unsuspected diagnoses, such as malignant conversion (Marjolin ulcer), detect the presence of biofilms, and facilitate quantitative culture. The best approach is to take one sample from the border and another from the bed of the ulcer.
Doppler Ultrasound (Echo Doppler)All patients with a venous ulcer should undergo Doppler ultrasound as this technique provides valuable information on involvement of the superficial, perforator, and deep veins, and whether reflux, obstruction, or both are present. This technique is essential for assessing whether the patient should be referred for vascular surgery.10
Nuclear Magnetic Resonance11When underlying osteomyelitis, a condition particularly common in patients with diabetes, is suspected, nuclear magnetic resonance is a particularly useful diagnostic tool. With a specificity of almost 100% and a sensitivity of 90%, this technique is far superior to conventional radiography. It is also replacing arteriography for the assessment of arterial blood flow when the patient has a very low or very high ankle-brachial index (this application is known as magnetic resonance angiography).
Other procedures, such as venography and intravascular ultrasound should, we believe, be left to the discretion of the vascular surgeons.
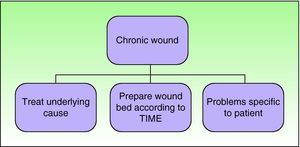
TreatmentThe treatment algorithm shown in Figure 7 is valid for any type of ulcer. The first step is to treat the cause of the ulcer, if possible. Appropriate treatment would be immunosuppressive agents for vasculitis, surgery for a tumor, compression therapy for a venous ulcer, and orthopedic therapy to avoid direct pressure in the case of an ulcer associated with diabetic foot. In addition, we should bear in mind general patient characteristics, such as nutritional status, social problems, and psychological state. These are all important in wound healing. Several studies have shown that delayed wound healing can be associated with stress and high levels of adrenaline.12,13 One of the mechanisms by which this occurs is thought to be activation of β-adrenergic receptors present in the keratinocytes. This would justify the use of β-blockers in these patients, although the role of such agents in this field is still under study.14
A basic issue addressed in this review, and one that applies to any chronic wound, whatever the site, is the optimum preparation of the ulcer bed, that is, how can we provide the most appropriate environment for the ulcer to heal as fast as possible. Since the study by Winter15 in 1962, we know that a moist environment accelerates healing.16 This discovery revolutionized wound management, and increasingly sophisticated wound dressings were developed. A powerful sector emerged within the pharmaceutical industry aimed almost exclusively at nursing staff. Table 3 shows the various types of dressings that have been used, starting from the early dressings made of polyurethane films with little capacity for exudate retention, up to the dressings currently available. The table is not an exhaustive list, but rather shows those dressings that the author uses on a regular basis.
Main Types of Dressing and Their Indications.
| Trade Name | Indication | Remarks | |
| Hydrocolloids | Varihesive gel controlComfeel | Ulcers with scant exudationNeed for autolytic debridement | Formation of foul-smelling and pseudopurulent gel |
| Foams | Perma foam, Tielle, Biatain, AllevynMepilex, VersivaXC | Ulcers with moderate exudation | Comfortable for the patient |
| Alginates | Sorbsan, Urgosorb Algosteril | Ulcers with abundant exudationBleeding ulcers | Cut to ulcer size |
| Hydrofibers | Aquacel | Ulcers with abundant exudation | More absorbent dressings |
| Extra Fine | Silicone:MepitelHydrocolloids:Varihesive extra fineUrgotul | Epithelized ulcerDonor zonesEpidermolysis bullosaNeurodermatitis | |
| Hydrogels | Varihesive hydrogelNu-gel PurilonHydrosorbNormlgel | Dry ulcersAutolytic debridement | Need secondary dressing |
| Silver Dressing | Foams: Mepilex AgBiatain AgHydrofibers: Aquacel AgActivated carbon: Actisorb plusGauzes: Atrauman, Acticoat | Infected ulcers or those with critical colonizationPresence of biofilms | Use for limited time |
| Others | Honey: MelmaxIonically charged: TrionicIbuprofen: Biatain-ibuMMP binding agents: PromogranMMP inhibitors, TIMP stimulators: Catrix | Treatment-refractory ulcers |
The TIME acronym (tissue removal, infection control, moisture balance, and edge of the wound) was coined by Falanga et al17 to remind us of all the aspects that need to be analyzed in the management of any ulcer, and to assist us in making appropriate decisions and preparing the wound bed for healing.
- -
Tissue removal. Removal of nonviable tissue (necrotic tissue) is necessary to reduce the risk of infection and to eliminate senescent cells, proteolytic enzymes (such as matrix metalloproteases [MMPs], the overactivity of which affects healing, particularly in the case of MMP-2 and MMP-9),18 and corrupt extracellular matrix.19 The first step is to clean the ulcer by gently rinsing with saline solution. Traditional antiseptics, such as chlorhexidine, hydrogen peroxide, or povidone iodine, should not be used as these are toxic for keratinocytes and fibroblasts in vitro. Although the question is still open to debate, the occasional use of antiseptics is considered acceptable to reduce the bacterial load.20 However, in such circumstances, guidelines recommend the use of the less toxic antibacterial agents, such as dressings impregnated with silver, cadexomer iodine, or polyhexanide (Table 4). After cleaning, if necrotic tissue is present, one of the following debridement procedures should be chosen (Table 5). If there is abundant necrotic tissue, the method of choice is surgical debridement with scissors or a scalpel because such methods can be performed quickly. However, arterial ulcers should not be debrided as ischemia increases the risk of infection, although debridement of such ulcers can sometimes be performed in the operating room. In the event of eschar of ischemic origin on the heel or neck, the best approach it to leave the eschar and not use surgical techniques. In this case and in others where surgical debridement is ruled out, we use enzymatic debridement. The most widely used agent in Spain is a collagenase ointment. This method is slow and not particularly selective, particularly when papain is used, and it can also cause pain. In the case of very dry eschar, a scalpel can be used to make incisions to facilitate the penetration of the ointment.2 In autolytic debridement the ulcer is simply maintained in a moist environment to facilitate the elimination of necrotic debris by the body's own macrophages. This approach is indicated when there is little necrotic material or for maintenance. If the ulcer bed is dry, extra moisture should be applied, for example through the use of hydrogels.21 Other less widely used debridement methods are presented in Table 6.
Table 5.Debridement Methods.
Type Characteristics Enzymatic Slow, nonspecificMake cuts in extensive escharMay be painful Surgical Fast, nonspecificMethod of choice for extensive escharNot suitable on heels or neck Biological (using Phaenicia sericata larvae) Very specificNot available in Spain MechanicalWet to dry dressingAspiration systems NonspecificPainful Autolytic Specific, slowMethod of choice if there is limited slough Table 6.Hypotheses Proposed to Explain Chronic Ulcers.
Theory Therapeutic Focus High bacterial load or formation of biofilms Silver dressing, lactoferrin gel, negative pressure therapyQuorum sensing inhibitors Disorganized or corrupted extracellular matrix Artificial skinAmelogenin Excessive metalloproteinase activity Metalloproteinase inhibiting dressingCollagen powder Decrease in growth factors Application of recombinant factorsPlatelet-rich plasmaGene therapy Excessive activity of proinflammatory cytokines Blockade of implicated cytokines (anti-TNF agents, etc) Cells with senescent phenotype Stem cell therapies Abbreviation: TNF, tumor necrosis factor.
- -
Infection control. Almost all ulcers are contaminated by bacteria. However, since the bacteria do not proliferate and their presence does not interfere with healing, routine cultures are not useful. The term colonization is used when the bacteria replicate and influence the healing process but do not invade adjacent tissues or induce signs of infection. Critical colonization refers to a situation in which the bacteria proliferate to such a degree that they start to have an impact on the healing process. The signs of critical colonization include the following: friable, grayish granulation tissue; absence of granulation tissue; increased exudate production without accompanying edema; discharge with an abnormal smell; increased pain compared to previous dressing changes; failure to improve despite correct treatment. The ulcer is said to be infected when the number of bacteria exceeds 100 000 per gram of tissue. Infection is usually accompanied by other signs, such as fever, enlarged lymph nodes, cellulitis, or purulent exudate. Samples should be taken for culture only when critical colonization or infection is suspected. Quantitative cultures are preferred, as discussed above, and treatment in the form of nontoxic antiseptics and empirical oral antibiotics can be given while waiting for the results of susceptibility testing. As is the case with traditional antiseptics, most experts do not recommend the use of topical antibiotics. In recent years, there has been much debate about the mechanisms by which bacteria can influence healing in the absence of clinical infection. Currently, most experts agree that the main mechanism by which healing is delayed is through the formation of biofilms.22 Biofilms are aggregated bacterial colonies encased in an extracellular matrix of polysaccharides that protects them against antibiotics and the immune system. Bacteria in these biofilms can produce substances that inhibit the proliferation of keratinocytes, such as lipopolysaccharides in the case of Pseudomonas aeruginosa,23 or induce the accumulation of polymorphonuclear cells and their MMPs.24 Biofilms are implicated in a number of pathologic processes in addition to chronic wounds, such as for example the formation of bacterial plaque in the oral cavity, chronic otitis media, cystic fibrosis, and chronic prostatitis.25 The means by which bacteria communicate to initiate expression of the genes that determine the synthesis of the matrix is known as quorum sensing. Intensive research into this process is underway with the hope that it will be possible to inhibit this intercellular communication. The presence of biofilms in an ulcer can be demonstrated by biopsy.26 They are found in 60% of chronic ulcers to a greater or lesser extent,27 compared to 6% of acute ulcers. The question is what to do when the presence of biofilms in the ulcer is suspected or confirmed. While we await the development of quorum sensing inhibitors, it has been shown that debridement, cadexomer iodine, lactoferrin gel, silver dressings, negative pressure therapy, and even honey are active against biofilms.28
- -
Moisture balance. In line with the rationale for wet dressings discussed earlier, we should choose a dressing that retains part of the exudate in the ulcer bed, while avoiding maceration at the edge of the lesion. As shown in Table 4, several types of dressing are currently available for an uncomplicated ulcer.
- a.
Hydrocolloids. These dressings are coated with a combination of 3 hydrocolloids, for example sodium carboxymethylcellulose, pectin, and gelatin. Since they are fairly occlusive, their use is not recommended on infected ulcers. They are only moderately absorbent and can therefore be used in mildly exudative ulcers or when it could be useful to maximize autolytic debridement. One of the characteristics of this type of product, which can limit its use, is the formation of a viscous, pseudopurulent, and foul-smelling gel that can give the impression of infection if the physician is not aware of this possibility. These dressings have now been largely replaced by foam products.
- b.
Polyurethane foams. Hydrocolloids have largely been replaced by polyurethane foams in mildly or moderately exudative ulcers, not because foam dressings are any more effective but because they are more comfortable. Foam dressings are made from a combination of polyurethanes, acrylates and other substances and comprise an hydrophobic outer layer and a hydrophilic and absorbent inner layer. Adhesive and nonadhesive dressings are available. They are semiocclusive, and can be used on infected ulcers.
- c.
Alginates. Alginates are made of sodium alginate extracted from brown seaweed. They are extremely absorbent and are used in highly exudative ulcers. They also have hemostatic properties, making them useful in patients receiving anticoagulant therapy who have bleeding ulcers or after debridement. They should be cut to the shape of the ulcer as they can macerate the edge and, as they are not adhesive, a secondary dressing is often required.
- d.
Hydrofibers. Hydrofiber dressings are formed from microfibers of pure carboxymethylcellulose. These are the most absorbent dressings and they are used in the most exudative ulcers. They are not adhesive or impermeable so a secondary dressing is required.
- e.
Hydrogels. Hydrogels are preparations with a high water content and microcrystalline systems of polysaccharides and synthetic polymers. They come as gels or sheets, and are minimally absorbent. For this reason, they are used in dry ulcers and to facilitate autolytic debridement. The gel, which is the most widely used form, requires a secondary dressing.
- f.
Other dressings for consideration include the following: those containing MMP binding agents, although the in vivo efficacy of these products has yet to be demonstrated; honey dressings; silicone sheets; and extra fine foam dressings or hydrocolloid dressings, which are useful in epitheliating ulcers or for graft donor areas.
The reader should be aware that it is impossible to review all the dressings available and that there are very few studies to guide usage.29
Another aspect to take into account is the size of the dressing and the interval between changes. Except for alginate dressings, which have to be cut to fit the ulcer because the wound edges tend to become macerated, the dressing should extend beyond the ulcer edges by several centimeters. The interval between changes will vary according to how much exudate is produced. The exudate should not be allowed to seep through the dressing as this would render it ineffective. In general, with an appropriately absorbent dressing, we can wait 2 to 3 days between changes.
- a.
- -
Edge of the wound. The edge of the wound should be kept in optimal conditions for healing.30 One technique used is to prevent maceration by applying a water-based paste at the edge of very exudative ulcers. Another approach is to debride the edges of the ulcer if they become sclerotic (a common problem in longstanding ulcers and those associated with diabetic neuropathies); in such cases we make an acute wound in a chronic ulcer to accelerate healing. If dermatitis is detected in the surrounding skin, a topical corticosteroid should be prescribed. These agents are also very useful when abundant granulation tissue protrudes beyond the edges of the ulcer; the application of a topical corticosteroid during 2 or 3 dressing changes will flatten the lesion in all cases.
This section deals with pharmacological treatments that accelerate healing and compression therapy, an essential component in the management of venous ulcers.
- a.
Drugs in the treatment of chronic wounds. The use of drugs to promote wound healing is currently limited. Pentoxyfylline appears to speed up the healing of venous ulcers when it is administered together with compression therapy.31 Fibrinolytic and antithrombotic effects and inhibition of proinflammatory cytokines are some of the pharmacological mechanisms that could explain the action of this agent. Other potentially useful drugs include retinoic acid, applied topically at concentrations of 0.025%-0.05% to the granulation tissue to stimulate angiogenesis,32 and β-blockers to block the effect of stress-related adrenaline release on keratinocyte migration.33 However, there is little solid evidence to recommend their widespread use.
- b.
Compression therapy. Compression therapy is the treatment of choice for venous leg ulcers.34 Unless contraindicated, it should always be used to avoid delayed venous ulcer healing. The aim of this therapy is to reduce edema and distension of the superficial venous system and to reinforce the effect of the calf muscle pump on the deep venous system. In addition, it may reduce the presence of proinflammatory cytokines in the exudate.35 There are two distinct types of compression therapy. Containment therapy, which uses short-stretch bandages, only prevents expansion of the calf when walking. This approach is not useful in patients confined to their beds as no pressure is exerted when the patient is at rest. True compression therapy, on the other hand, uses elastic systems. The pressure is governed by the Laplace law, which states that the pressure exerted on a cylindrical structure is proportional to the external pressure and inversely proportional to the radius of the structure (P=T/r). Before compression therapy is prescribed, the patient's ankle-brachial pressure index should be calculated using a Doppler ultrasound; if this index is less than 0.6, compression therapy should not be applied without consultation with a vascular surgeon. If the index is between 0.6 and 0.8, short-stretch systems should be used. For values greater than 0.8, compression therapy can be used. If, however, the index exceeds 1.2, calcification may be present, particularly in patients with diabetes, and magnetic resonance angiography should be ordered. Compression therapy can be applied with either elastic bandages or elastic stockings. For these to be useful in ulcer healing, they should apply a pressure of more than 35-40mmHg to the ankle. The conventional type of elastic bandage always has a layer of cotton in contact with the skin. The alternative is a multilayer system, in which different types of bandages (2 to 4 layers) are applied in a specific order to ensure appropriate pressure is applied. The multilayer system is more expensive, but it is simpler to apply and makes it easier to achieve the correct pressure. It is also more effective in my opinion. The drawback of using bandages is that the patient has to keep them on until the next dressing change, and trained personnel are required to achieve appropriate compression. Elastic stockings, on the other hand, have the advantage that patients can take them off at night and put them on in the morning before they start walking, although this requires a degree of skill and physical strength. Special shoehorns can, however, make it easier to put on the stockings. Elastic stockings can be classified according to the pressure they exert. Strong or grade III compression (pressure between 25-40mmHg) is indicated for active ulcers, whereas medium or normal compression, that is grade II compression (pressure between 18-25mmHg), is indicated for preventing recurrence. If the limb is deformed in the shape of a champagne bottle because of the presence of lipodermatosclerosis, elastic stockings are not useful and several layers of cotton bandage should be wrapped around the narrowest part of the limb to give it a cylindrical form before applying an elastic bandage.11 A Cochrane meta-analysis showed that compression therapy is more effective than no therapy and that multilayer systems are superior to conventional bandages.36
Between 10% and 20% of ulcers do not progress favorably notwithstanding appropriate diagnosis and treatment and even when treated in chronic ulcer units. There is no generally accepted explanation for this failure to heal, although several hypotheses have been put forward (Table 6). Some of these, such as the formation of biofilms, have been discussed earlier, and others are briefly discussed below, along with therapeutic approaches that have been proposed to address these issues.
Excessive Metalloproteinase ActivityIn any healing process, MMPs and tissue inhibitors of metalloproteinases (TIMPs) must be present at the right time and in appropriate quantities to enable such important processes as collagen remodeling, angiogenesis, and epithelization. Studies in rats and humans have shown excess MMPs to be present in chronic ulcers of differing etiologies, although the MMPs implicated vary. In diabetic foot ulcers, increased MMP-1 and 8 and decreased TIMP-1 were reported in 1 study37 while increased MMP-1, 2, 8, 9, and 14 and decreased TIMP -2 were reported in another.38 In pressure ulcers, increased MMP-9 and decreased TIMP -1 have been found.39 It is not clear how excessive quantities of MMP hinder wound healing, although possible mechanisms include collagen degradation and deactivation of growth factors. Many MMPs are produced by inflammatory cells, and hence the excess in MMPs could be the result of the marked inflammation present in chronic ulcers. In fact, tumor necrosis factor-α (TNF-α) can activate MMPs and inhibit TIMPs,35 as discussed below. Since the discovery of the possible role of MMPs in chronic ulcers, inhibition of these enzymes has been considered as a possible pharmacological target. Complete blockade is not desirable because these enzymes play a fundamental physiological role in healing. The current approach is to try to eliminate excess activity through use of dressings that bind MMP, although the effectiveness of this approach has yet to be demonstrated. In this context, the use of collagen powder deserves a mention. This agent has been used for more than 30 years in the management of chronic ulcers. Initially, its action was attributed to stimulation of the activity of fibroblasts and keratinocytes, but recent studies have shown that it can also stimulate TIMPs and inhibit MMPs.40
Growth Factor DeficiencySeveral growth factors intervene in normal healing processes. These include fibroblast growth factor, epidermal growth factor, hepatocyte growth factor, platelet-derived growth factor (PDGF), and transforming growth factor (TGF) β.41 The general function of these factors is to promote proliferation of the cells responsible for healing, and so deficiency in these factors would hinder the process. However, some of these factors, such as TGF-β, appear to inhibit epithelization, which would mean that an excess of this factor could be harmful. Their functions overlap in some cases, and they appear sequentially during the healing process. They must therefore be present in the correct quantities and at the right time if the wound is to heal. Currently, the only growth factor used in clinical practice is PDGF for the treatment of diabetic foot ulcers. The benefit is limited in relation to the cost of treatment. Other approaches are not to use growth factors in isolation but rather to opt for sources of multiple factors, such as platelet rich plasma.42 Alternatively, more effective delivery methods may be sought, such as gene therapy with viral vectors and transient liposome systems.43 Studies with knock-out mice lacking the genes coding for individual growth factors are shedding light on the importance of each factor and should, in the near future, enable the design of more effective strategies for the use of these agents.44
Increased Proinflammatory CytokinesAnother typical phenomenon in chronic ulcers is an increase in the quantities of proinflammatory cytokines, such as IL-1, IL-6, and TNF-α.45 If, as would appear to be the case in a number of studies, an excess of these cytokines can delay wound healing, chronic ulcers could be classified as inflammatory diseases and so benefit from the advances that have been made in the treatment of such diseases with biologic agents. TNF-α is a cytokine that has attracted much interest in this setting. When present at low concentrations, it can have a beneficial effect on wound healing by promoting collagen synthesis and fibroblast proliferation, but at high concentrations, it may inhibit collagen synthesis and angiogenesis, and enhance MMP function.46 In fact, TNF-α is 100 times more abundant in nonhealing venous ulcers than in acute wounds and it also becomes less abundant as an ulcer heals in response to compression therapy.35 TNF-α is actually being considered as a therapeutic target in these patients. In one study, etanercept was shown to reduce the cytotoxic effect of chronic wound fluids on fibroblasts.47 In another study, topical infliximab was applied to 14 ulcers of different types; 5 of the 14 ulcers had healed at week 8, and a 75% improvement was observed in 4 others.48 However, these drugs should only be used with extreme caution when there is a risk of infection, as is the case in patients with leg ulcer. More specific TNF-α blockade might be achievable in these patients given that the negative effects on healing appear to be mediated by the p55 receptor and not the p75 receptor. In fact, mice lacking the p55 receptor showed accelerated wound healing.49
Cellular SenescenceThe different types of cells are programmed to replicate a certain number of times before entering into what is known as cellular senescence. At this point they cannot divide and their cell cycle is detained at G1. These cells accumulate certain markers such as senescence-associated β-galactosidase. Senescence is irreversible and controlled genetically by telomer length. Various studies of chronic ulcers have shown an increase in senescent cells, particularly among fibroblasts, but also among keratinocytes, endothelial cells, and macrophages.50 The presence of these senescent cells also could have a marked impact on the healing capacity of the ulcer and could make the ulcer refractory to treatment with growth factors because such cells would not respond to these agents. The true importance of this phenomenon has yet to be established, but it could provide a rationale for strategies such as the application of nonsenescent cells in the form of stem cell therapy.30,44
Micrografts or artificial skin obtained from tissue engineering could also be considered. The approach of taking an autologous graft from donor skin of the patient and placing it over the ulcer has been used since the nineteenth century. In my opinion, this approach should be reserved for refractory cases with no sign of infection or critical colonization because the donor region will create new lesion with its own potential complications. Even in the best of cases, the percentage take-up of these grafts ranges from 15% to 50%.51 In actual fact, some authors wonder whether these grafts are not acting as crude sources of stem cells. The same could be said about the use of artificial skin. A review of these types of skin substitutes would go beyond the scope of this article, although there are some excellent articles on the topic.52 My impression is that these materials could be of use when new skin is needed quickly, for example in patients with extensive burns, toxic epidermal necrolysis, or epidermolysis bullosa. In patients with chronic ulcers, such products have been shown to be only slightly superior to traditional dressings while being much more expensive. In any case, future clinical trials should help define their utility.
Conflicts of InterestThe author declares no conflicts of interest.
Please cite this article: Velasco M. Aspectos diagnósticos y terapéuticos de las úlceras de las piernas. Actas Dermosifiliogr.2011;102:780-790.