Primary cutaneous lymphomas (PCLs) are a heterogeneous group of lymphoid tumors that originate primarily in the skin. Most PCLs (75%) are T-cell lymphomas and only 20% to 25% involve B cells. It is important to differentiate between cutaneous lymphomas and lymph node tumors given the differences in their molecular biology and clinical, histopathologic, and immunophenotypic features. Moreover, PCLs generally follow a more indolent course and require different treatments.
Many treatment options are available for managing PLC's. The choice should be based primarily on the clinical stage of disease but must also take into consideration other factors, such as the patient's age and general health, the availability and accessibility of the treatment, and the cost-benefit ratio. It will be important to use a multidisciplinary approach, involving a team of expert dermatologists, hematologist-oncologists, and radiotherapists who are familiar with this rare disease. Recent years have seen the emergence of many new therapies, particularly for advanced stages of the disease and for patients whose tumors have proven refractory to treatment. The objective of this article is to review all the treatment options available to us.
Los linfomas cutáneos primarios (LCP) constituyen un grupo heterogéneo de neoplasias linfoides que se originan primariamente en la piel. La mayoría (75%) son linfomas de células T y solo un 20-25% se originan a partir de los linfocitos B. Es importante diferenciar los LCP de sus equivalentes ganglionares, dado que presentan características clínicas, histopatológicas, inmunofenotípicas y de biología molecular diferentes con un pronóstico en la mayoría de los casos más indolente y tratamientos diferentes.
Existen múltiples opciones terapéuticas en el manejo de los LCP. La elección del tratamiento debe basarse principalmente en el estadio clínico del paciente, pero deben considerarse también otros factores, como la accesibilidad a los tratamientos, la edad y el estado general del paciente o el coste-beneficio. Además es importante el abordaje multidisciplinar de estos pacientes, formando un equipo experto entre dermatólogos, hematooncólogos y radioterapeutas que conozcan bien esta infrecuente patología. En los últimos años asistimos a la aparición de múltiples terapias nuevas, especialmente para el tratamiento de los estadios avanzados o de pacientes refractarios a tratamientos previos. El motivo de este artículo es revisar todas las alternativas terapéuticas a nuestro alcance.
Primary cutaneous lymphomas (PCLs) are a heterogeneous group of lymphoid malignancies that originate primarily in the skin.1 Most (75%) are derived from T cells (primary cutaneous T-cell lymphoma [CTCL]) while 20% to 25% are derived from B cells (primary cutaneous B-cell lymphoma [CBCL]). Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common such PCLs. It is important to differentiate PCLs from their equivalent lymph node disease, given that they show clinical and histopathologic differences and have different immunophenotypes and a different molecular biology. Most importantly, PCLs have a more indolent course in most cases and different treatment regimens are used.
For a correct diagnosis and hence appropriate treatment, it is necessary to be familiar with the latest classifications such as those of the European Organisation for Research and Treatment of Cancer (EORTC)/World Health Organization (WHO)1 (Table 1) and the WHO (2008).2,3 PCLs have a very low incidence,4 and most patients survive for a long time. It is therefore difficult to assess the impact of a given treatment on outcome and there are almost no case-control studies to guide us. The levels of evidence are therefore low. Ideally, all patients with PCLs would be included in a clinical trial.
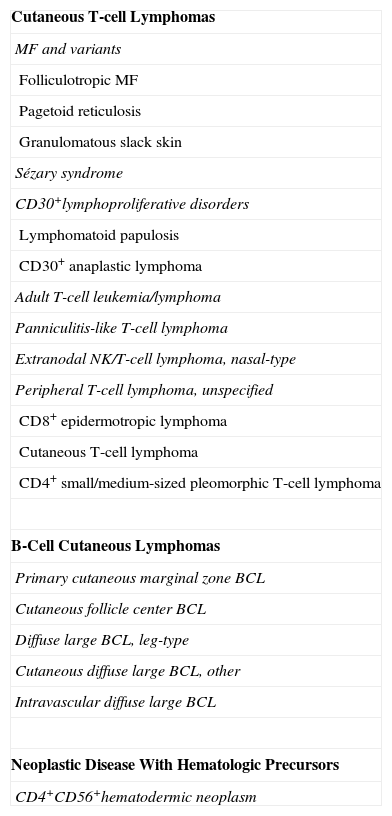
World Health Organization/European Organisation for Research and Treatment of Cancer Classification of Primary Cutaneous Lymphomas (2005).
| Cutaneous T-cell Lymphomas |
| MF and variants |
| Folliculotropic MF |
| Pagetoid reticulosis |
| Granulomatous slack skin |
| Sézary syndrome |
| CD30+lymphoproliferative disorders |
| Lymphomatoid papulosis |
| CD30+ anaplastic lymphoma |
| Adult T-cell leukemia/lymphoma |
| Panniculitis-like T-cell lymphoma |
| Extranodal NK/T-cell lymphoma, nasal-type |
| Peripheral T-cell lymphoma, unspecified |
| CD8+ epidermotropic lymphoma |
| Cutaneous T-cell lymphoma |
| CD4+ small/medium-sized pleomorphic T-cell lymphoma |
| B-Cell Cutaneous Lymphomas |
| Primary cutaneous marginal zone BCL |
| Cutaneous follicle center BCL |
| Diffuse large BCL, leg-type |
| Cutaneous diffuse large BCL, other |
| Intravascular diffuse large BCL |
| Neoplastic Disease With Hematologic Precursors |
| CD4+CD56+hematodermic neoplasm |
Abbreviations: BCL, B-cell lymphoma; MF, mycosis fungoides; NK, natural killer.
Many therapeutic options are available for the management of PCL.5,6 The choice of treatment should be based mainly on the clinical stage, but other factors should also be considered such as access to treatments, age and general health of the patient, and cost-benefit ratio. In the initial stages of the disease, no data are available to demonstrate the superiority of systemic treatment (the more aggressive option) over local treatment targeting the skin lesions. A conservative approach is therefore recommended.
The treatments can be divided into 2 broad groups:
- 1
Treatments that target the skin and act only on the population of neoplastic cells in the skin. In this case, the adverse effects are usually less severe than those arising with systemic treatments. This type of treatment is in practice the only one recommended for first-line treatment of early phases of MF. In advanced stages, it is still an important option, forming part of combination therapies. Such treatments include:
- -
Topical corticosteroids
- -
Alkylating agents: topical nitrogen mustard (mechlorethamine) and carmustine
- -
Narrow-band UV-B phototherapy, psoralens+UV-A (PUVA), and other types of phototherapy
- -
Radiotherapy (conventional and electron beam)
- -
- 2
Systemic treatments, which include biological response modifiers (such as retinoids, cytokines, immunotoxins and vaccines, monoclonal antibodies, and histone deacetylase inhibitors), chemotherapy (traditional single- or multiagent chemotherapy or polychemotherapy, new chemotherapies), and allogeneic stem cell transplantation.
Corticosteroids are able to induce apoptosis in most neoplastic lymphocytes in the skin and reduce the number of Langerhans cells, thereby interfering with the stimulation of neoplastic cells. Although such treatments have been in use for years, there are few studies of their use.7,8 Zackheim et al.8 reported their experience in 79 patients with MF in the form of patches or plaques. Patients received medium- and high-potency corticosteroids (class I-III), sometimes with an occlusive dressing. In patients with stage T1 disease, 63% had complete response (CR) and 31% had partial response (PR), while in patients with stage T2 disease, 25% had CR and 57% had PR. The adverse effects observed were adrenal suppression in 10 patients, skin irritation in 2, and skin atrophy with stretch marks in 1 patient. The authors concluded that the treatment is well tolerated and effective in the very incipient stages of the disease.
Alkylating AgentsAlkylating agents are drugs that act on different mechanisms and functions of DNA, thereby finally inducing cell death.
Topical Mechlorethamine (Nitrogen Mustard)Although the exact mechanism of action of mechlorethamine is unknown, when administered systematically, it acts as an alkylating agent with an antimitotic effect. Its topical activity, however, appears to be mediated by immune mechanisms or by interaction with Langerhans cells. The drug is usually used as an aqueous solution with a strength of 10-20mg/100mL or as a gel (10-20mg/100g petroleum jelly).9–15 An ongoing randomized, double-blind, multicenter trial is comparing the safety and efficacy of 2 preparations of nitrogen mustard (0.02% in propylene glycol and 0.02% in petroleum jelly) in patients with stage I or IIA MF refractory to topical corticosteroids.16 The preliminary results have shown similar efficacy (overall response rate [ORR] of around 70%) and a good safety profile (no systemic absorption was seen) for both preparations. In clinical practice, the drug is initially applied daily until the lesions have cleared (in 3 to 6 months with the solution and 6 to 12 months with the gel). An intermittent maintenance regimen is followed thereafter. Application to the entire body surface is usually recommended, though avoiding intertriginous areas. However, the need to treat unaffected areas is not very clear. Response varies between 50% and 75% in stage T1 and 25% and 50% in stage T2. Contact dermatitis (allergic or irritant) may arise, particularly when using the aqueous solution (30% vs <5% with the gel). Urticaria and anaphylactic reactions have also been reported. Prolonged use increases the risk of nonmelanoma skin cancer, particularly if the patients have received prior PUVA or total skin electron beam therapy. In general, myelosuppression or other systemic effects do not occur. Efficacy appears to be similar for the 2 preparations (solution and gel), although prospective studies comparing the 2, other than the one described above,16 have not been undertaken. In recent years, the availability of mechlorethamine in hospital pharmacies has been limited, and so it is unfortunately being used less and less despite its efficacy.
CarmustineCarmustine is an alkylating agent that induces cell death through inhibition of DNA synthesis. It is used as an alcohol solution (2mg/mL) or as an ointment.17–20 It should be applied daily to the lesioned skin, and the clearance time is similar to that of mechlorethamine. Response rates vary from 86% in stage T1 to 48% in stage T2. Most patients experience erythema, sometimes followed by persistent telangiectasia, and approximately 3% to 5% develop mild leukopenia due to myelosuppression. The agent can be used as an alternative in patients who are allergic to mechlorethamine, although the availability of carmustine is also limited.
PhototherapyNarrow-Band UV-B Phototherapy (311-312nm)Several studies have shown the usefulness of narrow-band UV-B phototherapy in the initial stages of MF (CR rates of around 75%, with a mean response duration of 51 months).21–26 The risk of skin cancer is increased, however, and therapy is only effective in MF with incipient lesions with little or no infiltration, given the limited penetration of the radiation. This type of therapy is considered particularly useful in those patients who do not tolerate psoralens, those with very light skin, and those with very incipient lesions.27
Psoralens Plus UV (320-400nm)PUVA is the classic treatment in the early stages of lymphatic disease.20,29–34 Usually, the patient starts with 2 to 3 weekly sessions, with the interval between sessions being reduced according to response. There is no consensus about whether it is necessary to follow a maintenance regimen once CR has been achieved.35 In a study of 82 patients with MF in the form of superficial plaques and/or palpable plaques, CR was obtained in 65% and PR in 30%, with a mean response duration of 43 months.28 Chronic actinic damage was observed in 10% of the patients, with the appearance of carcinomas in 6 patients: 3 with basal cell carcinoma and 3 with squamous cell carcinoma. Although there are studies that show a statistically significant increase in carcinogenesis, including melanoma, we believe that this risk is more theoretical than real, at least in this group of patients. According to the most recent studies, the disease-free interval is related to a higher cumulative dose of PUVA and longer treatment times.27,35 Although late recurrences are seen, between 30% and 50% of patients in stages IA, IB, and IIA can maintain CR for periods of up to 10 years. The disease-free survival at 5 and 10 years for patients in stage IA was 56% and 30%, respectively. For those patients in stages IB/IIA, 5- and 10-year disease-free survival was 74% and 50%, respectively. However, the overall survival did not vary significantly between those with recurrences and those without.
Others Types of Phototherapy- -
Extracorporeal photopheresis.36–39 In a modification of PUVA, after ingestion of psoralen, circulating mononuclear cells from the patient are exposed to UV-A. The main drawback of this approach is the high cost and, in addition, efficacy is no better than other treatments. The best responses are obtained in patients with early-stage SS who have normal CD8 counts and have not received prior aggressive therapies.
- -
Hypericin. This new photodynamic plant derivative induces T-lymphocyte apoptosis in association with visible or UV-A light. If preliminary results are confirmed, this option could be better than the other forms of phototherapy,40 as visible light does not increase the risk of skin cancer.
- -
Photodynamic therapy. The use of photodynamic therapy with derivatives of 5-aminolevulinic acid could be particularly appropriate in patients with few lesions or lesions on the scalp.41
- -
Monochromatic excimer laser light. Excimer lasers at a wavelength of 308nm have also been used and are approved by the US Food and Drug Administration (FDA) for psoriasis and vitiligo. Passeron et al.42,43 have performed a clinical trial in which good responses were obtained in very incipient lesions. This approach can therefore be considered in areas that are not usually accessible with phototherapy.
The evidence to support these approaches is, however, limited. Likewise, there is little evidence with regard to other therapeutic options.
RadiotherapyConventional (Orthovoltage)Conventional radiotherapy is used for highly infiltrated plaques or localized MF tumors refractory to other treatments.44 It is particularly indicated as front-line treatment of cutaneous marginal zone B-cell lymphoma and follicle center lymphoma along with excision.45,56 The recurrence rate is higher if doses less than 30Gy are used. Local radiotherapy has also been used in solitary MF lesions with impressive results.47
Total Skin Electron Radiation (Electron Beam Therapy)An electron beam is the treatment of choice for patients with MF with infiltrated plaques or small generalized tumors. The most widely used voltage is 6 MeV, and therapy is applied 4 days per week for a total dose of 3600 cGy in 10 weeks.48 The treatment can be repeated several times if reduced doses are used. Adverse effects include generalized erythema, edema, scaling and exudation, apparent worsening of existing lesions, total loss of skin appendages, transverse melanonychia and, less frequently, blistering. Hair loss is usually reversible if the total dose administered has not exceeded 2500 cGy. In the longer term, patients may develop edema, hyperpigmentation, telangiectasia, and persistent xerosis, while men may become sterile.48–50
The disease-free interval can be extended according to some authors by subsequent maintenance therapy, either with mustard derivatives or PUVA.48,49,51,52 The recommendations of the EORTC on total skin electron radiation in MF were published in 2002.53 The procedure is not recommended for erythrodermic CTCL (T3) given the risk of severe scaling and because the CR rates are higher in the earlier stages (CR of 90% in T1 and 70% in T2 disease) than in the more advanced stages, where the effect is only palliative.
It is known that CR rate depends on the stage of the disease, the dose applied, and the electron energy.51 Overall, CR rates of 96% are obtained in patients with stage IA, IB, and IIA disease; of 36% in those with stage IIB disease; and of 60% or less in patients with stage III disease. CR rates are also related to total dose (32-36Gy) and electron energy (4-6 MeV).52,53
Systemic TreatmentsBiological Response ModifiersRetinoidsIsotretinoin and etretinate have been used in the treatment of MF to similar effect. Their usefulness as monotherapy is limited, as it was seen that atypical cells were still present according to the pathology study even though the lesions had apparently healed. These agents have been combined with PUVA and interferon alfa (IFN-α). When combined with PUVA, they seem to be able to reduce the UV-A dose while with IFN-α they seem to enhance the response to interferon in early but not late disease stages. However, there are no randomized studies that compare IFN-α and IFN-α in combination with retinoids.54
Bexarotene is a new retinoid (more specifically a rexinoid) whose exact mechanism of action is unknown although it inhibits growth of tumor lymphocytes and also enhances apoptosis in vitro.55 Bexarotene selectively activates RXR receptors (a type of nuclear receptor activated by retinoic acid), acting as a regulator of cell differentiation and proliferation. In 2002, the FDA approved the compound, both for topical use as a 1% gel (which has so far not been marketed in Spain but can be obtained as an imported medicine) and for systemic use at an optimum dose of 300mg/m2/d. Regular laboratory testing should be performed during treatment, focusing mainly on triglyceride levels and thyroid hormones. Bexarotene induces hypertriglyceridemia, which can be marked at times. Preventive treatment should therefore be given the preceding week with statins or a fenofibrate but not with gemfibrozil as this fibrate increases the levels of bexarotene in blood, probably through inhibition of cytochrome P450. Hypothyroidism is another common adverse effect that can be controlled with administration of thyroid hormone. Bexarotene can be combined with other treatments such as PUVA or IFN-α. It may also find uses in a maintenance regimen after more aggressive therapies in patients with more advanced disease. As with other retinoids, it requires contraceptive measures in view of the risk of malformations.56–61
Interferon-alfaIFN-α is used as monotherapy administered subcutaneously (although intramuscular and intralesional administration is also possible) at a dose of 3 to 20 million units per day, 3 days a week, with a good (dose-dependent) response, particularly in early-stage disease (ORR of approximately 70%) or in combination with other therapies such as PUVA (apparently the most effective combination), retinoids, or purine analogs (fludarabine) with apparent benefit (studies that compare these options with IFN-α monotherapy are lacking). Adverse effects include flu-like syndrome, gastrointestinal disorders, bone marrow suppression, and elevated transaminases. The agent can be used for years as maintenance in patients who showed a good response and are at risk of relapse, although there is a risk of developing autoimmune diseases such as diabetes mellitus, thyroiditis, or hemolytic anemia.62–64
ImmunotoxinsDenileukin diftitox (DAB389-interleukin 2 [IL-2]) is the first fusion cytotoxin approved by the FDA for the treatment of CTCL.65,66 It acts as a specific cytotoxin for cells that express the IL-2 receptor. It is obtained by expression in Escherichia coli of the product of fusion of the human IL-2 receptor genes with cytotoxic sequences of diphtheria toxin. The agent binds to the IL-2 receptor (CD25) of T lymphocytes, thereby inhibiting protein synthesis, and is administered intravenously at a dose of 9 or 18mg/kg/d in 5-day cycles every 3 weeks. It has been shown to be particularly effective in stage IIB MF (response rate, 38%). Transient flu-like syndrome is reported in 60% to 70% of the patients, a similar figure to that seen with IFN-α. Other adverse effects such as urticaria, anaphylaxis, or vascular leak syndrome have also been reported. Some patients developed anti-DAB389 or anti-IL-2 antibodies in the first cycle, but there was no correlation with either toxicity or response. Efficacy has been boosted in recent studies through the concomitant use of oral bexarotene, particularly in patients with low or nonexistent expression of the IL-2 receptor.67,68 Combination therapy enabled lower doses of the 2 treatments to be used. The agent has been used in monotherapy in CTCLs other than MF,69 and in combination with radiotherapy.70
Monoclonal AntibodiesAlemtuzumabAlemtuzumab is a humanized monoclonal antibody that targets the CD52 glycoprotein expressed on the surface of T and B lymphocytes, natural killer (NK) cells, and to a lesser extent on monocytes and macrophages. The mechanism of action has not been fully elucidated but involves direct complement-mediated cell lysis and also antibody-dependent cytotoxicity and apoptosis. In 2003, its usefulness was demonstrated in 22 patients with pretreated MF/SS, most of whom had advanced disease (86%≥stage III and 36% with B symptoms) and a general poor state of health. The ORR was 55% (32% CR) and the mean response duration was 12 months.71 Recently, these encouraging outcomes have been reproduced.72 Alemtuzumab-induced cytopenia is potentially the most serious complication reported. Although different degrees of anemia and/or thrombocytopenia may develop due to mechanisms that are not well understood, T- and B-cell lymphopenia is a generalized finding in all patients. This predisposes them to serious opportunistic infections (particularly by cytomegalovirus and Pneumocystis jirovecii).73 Alemtuzumab seems especially useful in the management of pruritus associated with the erythrodermic forms (SS).74,75
ZanolimumabZanolimumab is another monoclonal antibody, which targets the CD4 receptor expressed on T lymphocytes and macrophages. It interferes with T-cell activation by impeding the interaction of CD4 with class II molecules of the major histocompatibility complex and also induces cell lysis through antibody-mediated cytotoxicity rather than by complement-mediated effects as is the case with alemtuzumab.76,77 Zanolimumab has been shown to be effective in 2 phase II multicenter studies of 47 patients with heavily pretreated persistent and refractory CTCL (38 MF and 9 SS).78 The arms in which high doses of zanolimumab were administered (560mg/wk for early stages [MF, stages IB-IIA] and 980mg/wk for advanced stages [MF, stages IIB-IVB, and SS]) achieved an ORR of 56% with an impressive mean duration of response of 81 weeks (particularly in MF). Overall, zanolimumab has an acceptable safety profile with moderate adverse effects such as dermatitis, eczemas, and infections limited to the skin and upper respiratory tract despite the severe CD4 cell depletion associated with its use. No differences were observed in the incidence of infection between the 2 different dose groups. Although a more marked CD4 cell depletion was observed among patients treated at the higher dose (560-980 vs 280mg/wk), of note was that there were no statistically significant differences in CD4 cell recovery once treatment had finished. On the basis of these findings, a pivotal phase III study was started and is currently in progress.
RituximabRituximab is a chimeric (human-murine) anti-C20 monoclonal antibody that is used as monotherapy or in combination with other agents mainly for the treatment of systemic non-Hodgkin B-cell lymphoma. Several studies have found it to be effective when administered intralesionally in patients with CBCL (marginal zone and follicle center cell types).45,46,79,80 It is considered particularly useful in patients with multiple lesions or those with a high risk of recurrence, as well as in areas where we wish to avoid the unsightly sequelae of surgery and/or radiotherapy. It is also used in combination with cyclophosphamide, vincristine, and prednisone with doxorubicin (CHOP) for CBCL, leg-type.
Histone Deacetylase InhibitorsHistones are proteins present in abundance in cell nuclei, where they form the chromatin of eukaryotic cells along with other types of proteins and DNA. In recent years, several molecules within the pharmacologic group of histone deacetylase inhibitors have been developed, encouraged by different experimental findings that suggest that excess histone acetylation occurs in most cancers.81,82 Four histone deacetylase inhibitors have been studied in the treatment of CTCL: vorinostat, romidepsin, panobinostat, and belinostat.
VorinostatVorinostat has been authorized by the FDA for the treatment of skin manifestations of patients with CTCL which persist, progress, or recur after at least 2 systemic treatments.83 However, the drug has not been marketed in Europe. The indication was established after a phase II study of 74 patients with MF/SS.84 Most of these patients had advanced disease stages (61 patients with ≥IIB disease and 30 patients with SS, while 22 patients had clinically abnormal lymph nodes). All patients had received intensive treatment (96% with bexarotene, 63% with IFN-α, 61% with chemotherapy, 36% with photophoresis, and 31% with denileukin diftitox). The dose was 400mg/d, administered orally, with adjustment or suspension according to toxicity or adverse effects. Overall, 29.7% achieved response. Among patients with advanced disease (IIB or worse), 29.5% achieved response. Among patients with SS, 33.3% achieved response. Vorinostat was generally well tolerated and the adverse effects observed most frequently were gastrointestinal (nausea, diarrhea) and constitutional (asthenia, anorexia, and weight loss). Cytopenias (thrombocytopenias and anemia) were also frequently reported. Neutropenia was not observed. Fewer than 15% of the patients required a reduction in vorinostat dose and 11% had serious adverse effects, which were mainly thromboembolic phenomena. QT prolongation was only observed in 3 patients.
Recent studies are using these agents in combination with other therapies such as bexarotene85 or IFN-α.86
RomidepsinRomidepsin is a new and potent histone deacetylase inhibitor that has been used in patients with CTCL and peripheral T-cell lymphomas. Several studies have demonstrated its usefulness,87–90 with an ORR of 41% (7% with CR and 33% with PR), a median response duration of 14.9 months, and a median time to progression of 8.3 months. The most frequently observed adverse effects were nausea, asthenia, and vomiting. Adverse effects grade 3 or worse were only observed in 33% of the patients. The most frequently observed adverse effects were disease progression (6%), fever (3%), sepsis (2%), tumor lysis syndrome (2%), and hypotension (2%). Six patients died, one possibly of treatment-related causes. QT prolongation was only observed in 2% of the patients (patients with significant cardiac abnormalities and/or those in treatment with QT prolonging agents or inhibitors of cytochrome P3A4 were excluded from the study).
PanobinostatThe new histone deacetylase inhibitor, panobinostat, not only produces histone acetylation but also induces p21, cell cycle arrest, apoptosis, and HSP90 (pan-histone deacetylase inhibitor) acetylation. Its usefulness and safety profile have been demonstrated recently in a phase II study performed in 40 patients with CTCL refractory to at least 2 prior treatments (mean of 5 prior treatments per patient).91 Patients received 20mg/d of panobinostat orally on days 1, 3, and 5 of each week until disease progression or intolerance. The response rates were poor. In patients who had received prior treatment with bexarotene (group 1, 25 patients), PR was obtained in 3 and 4 had stable disease. Three patients had progression. Thirty patients could not be evaluated because of limited follow-up at the time of publication. The most frequently observed adverse effects were diarrhea, thrombocytopenia, fatigue, asthenia, hypertriglyceridemia, taste alterations, nausea, and pruritus (15% of all patients). No significant QT prolongations were observed.
BelinostatBelinostat is another pan-histone deacetylase inhibitor that has been tested in a phase II clinical trial with 29 patients (15 with MF, 7 with SS, 5 without either MF or SS, and 2 with unclassified disease).92 The participants received 1000mg/m2 in a 30-minute infusion from day 1 to 5 of each cycle, every 3 weeks. Seventeen patients achieved stable disease for longer than 127 days. There were 2 PR and 2 CR with a median response duration of 273 days. It is important to note that the time to response was very short: 16 days (range, 14-35 days) with a substantial improvement in pruritus. There were no reports of grade 4 hematologic toxicity or cases of grade 3 QT prolongation. Four grade 3 or 4 adverse effects were observed: pruritus, erythema, edema, and adynamic ileus.
OthersBortezomibBortezomib is a drug indicated as first-line treatment in patients with multiple myeloma who are not candidates for hematopoietic stem cell transplantation and in patients with a relapse after prior treatment. The group at the Hematology Institute of the University of Bologna in Italy conducted a phase II study of 12 patients (10 with advanced MF and 2 with peripheral T-cell lymphomas with isolated cutaneous involvement).93 They reported an ORR of 67%, including 2 CR (17%) and 6 PR (50%). One patient with MF and 1 with peripheral T-cell lymphoma achieved CR (10% and 50%, respectively). CR lasted for more than 1 year after the last study dose in the patient with MF while relapse was observed 10 months later in the patient with peripheral T-cell lymphoma. The regimen of bortezomib used was the same as for multiple myeloma: 1.3mg/m2, given intravenously on days 1, 4, 8, and 11 of each 21-day cycle up to a total of 6 cycles. The adverse effects observed most frequently were neutropenia (2 patients [17%], WHO grade 3), thrombocytopenia (2 patients [17%], WHO grade 3), and sensory neuropathy (2 patients [17%], WHO grade 3). No infections or treatment-related deaths were reported during the study. The rationale for using bortezomib is that it can act as an inhibitor of nuclear factor κB (NF-κB), which is constitutively activated in CTCL cell lines, and induce apoptosis.94 NF-κB is a transcriptional factor implicated in the generation of inflammatory responses, regulation of the cell cycle, and protection against apoptosis.
LenalidomideThe antitumor effects of lenalidomide are attributed to several mechanisms of action: inhibition of the production of proinflammatory mediators by monocytes (tumor necrosis factor α, IL-1, IL-6, IL-12), enhancement of IL-2 and IFN-γ production by T lymphocytes, and enhancement of the cytotoxic activity of these cytokines and NK cells. In a phase II study, lenalidomide was administered at a dose of 10 to 25mg/d for 21 days in cycles of 28 days to 25 patients with extensively pretreated CTCL (mean of 6 prior regimens).95 Seven patients achieved PR after a mean of 9 cycles of treatment. The adverse effects observed most frequently were anemia, scaling, pruritus, and leg edema. In the high-dose group, intense neutropenia was observed in 2 patients while 1 patient discontinued treatment due to dysarthria.
ChemotherapyChemotherapy should only be used in CTCL in advanced stages of the disease, as it is no more effective than conservative treatment in the early phases. Almost all chemotherapy agents used for systemic lymphomas have also been used in advanced CTCL: alkylating agents, methotrexate, cisplatin, etoposide, bleomycin, vinblastine, cyclophosphamide. It is not clear whether one agent is any better than another and, in general, responses are short-lasting. The most effective combination, and therefore the most widely used one, is CHOP or CHOP without doxorubicin (CVP), but randomized studies have not been undertaken that demonstrate increased survival with any of these regimens.
MethotrexateMethotrexate is the first-choice treatment in CD30+ CTCL, particularly when the patient has multiple lesions (radiotherapy is the best alternative in solitary lesions).96 High weekly doses are required in some cases to achieve CR, with a higher risk of adverse effects.
Recently, 2 other chemotherapies (gemcitabine and pegylated liposomal doxorubicin [DOX-PEG]) have been shown to be useful in monotherapy.
GemcitabineGemcitabine is a pyrimidine antimetabolite indicated for solid tumors traditionally considered resistant to conventional treatment such as lung cancer, ovarian cancer, pancreatic cancer, and urinary bladder cancer. Its effectiveness in cutaneous lymphomas has been investigated in 2 studies. The first to these was a series of 32 patients (mostly with MF).97 CR was achieved by 22% (7 patients) while PR was achieved by 53% (17 patients). Unfortunately, the study used standard criteria for assessing response as if the patients had common forms of non-Hodgkin lymphoma and not specifically cutaneous lymphoma (severity-weighted assessment tool). Response was achieved by 73% of those with MF (26 patients), with 23% with CR and 50% with PR. The only patient included with SS did not respond. The median duration of CR was 10 months (range, 4-22 months). Gemcitabine was administered as an intravenous infusion at a dose of 1200 mg/m2 for 30minutes in cycles of 28 days (total of 6 cycles) on days 1, 8, and 15 of each cycle. The most frequently reported adverse effects were cytopenias. Reversible liver toxicity was the most frequently reported nonhematologic adverse effect (13 patients, 40%). No treatment-related deaths were reported. The second study was a series of 33 patients, most of whom had heavily pretreated MF (median of 5 prior regimens) (31 patients).98 Two patients had anaplastic CD30+ T-cell lymphoma. Compared to the first study, a higher dose of gemcitabine was used (1000mg/m2) but the schedule of administration and number of cycles were the same. Response was assessed using variables that included the area of skin involved, size of lymph nodes, and peripheral blood cytometry. Response was achieved by 68% of the patients (2 with CR). Myelosuppression was the most frequently observed adverse effect (grade 3 in 8 of the 33 patients) and 2 characteristic uremic hemolytic syndromes were diagnosed in patients with SS. Other adverse effects were elevated transaminase liver enzymes, mucositis, lethargy, fever, hyperpigmentation, infusion-related maculopapular rash, and different types of cardiovascular events. Another more recent multicenter study reported similar outcomes to the studies already discussed, but with significantly greater toxicity.99
Pegylated Liposomal DoxorubicinDOX-PEG is approved for the treatment of advanced ovarian cancer, relapsed multiple myeloma after hematopoietic stem cell transplantation, and AIDS-associated Kaposi sarcoma. The usefulness of DOX-PEG has also been demonstrated in patients with CTCL in observational and retrospective studies. Recently, a prospective, multicenter study analyzed use of this agent in 25 patients with MF and/or SS in stages ≥II refractory to at least 2 prior lines of treatment and in patients with CD30+ large-cell CTCL.100 DOX-PEG was administered intravenously every 4 weeks at a dose of 40mg/m2/d for up to 8 cycles. An objective ORR of 56% was reported (14/25 patients with CR and 9/25 with PR). Response rates were high in patients with SS, with 6 of the 10 (60%) responding. One of these responses was a CR. Response rates were also high in transformed CTCL, and 50% of these responses were a CR. In general, more adverse effects were seen than in the previous studies, with 4 episodes of serious infection and some cardiac events. DOX-PEG has also been shown to have good activity in 5 CBCLs (1 with marginal zone disease and 4 diffuse large-cell lymphomas, leg-type) in an Italian phase II pilot study (dose of 20mg/m2 every 3 or 4 weeks). An impressive prolonged CR was obtained in 4 of these patients (80%).101 In another study published recently, excellent outcomes were obtained with DOX-PEG in combination with bleomycin, vinblastine, and dacarbazine (CBVD regimen) in 37 patients with advanced PCL, both of T-cell origin (19 patients) and B-cell origin (18 patients).102 CR rates of 88.8% and 100%, respectively, were obtained. Between 4 and 6 cycles of CBVD were administered in the group of CTCL whereas those with CBCL received between 2 and 6 cycles (with rituximab added to the chemotherapy: R-CBVD). The safety profile was good. Subsequent allogeneic hematopoietic stem cell transplantation was performed in 3 patients (2 with CTCL and 1 with CBCL).
Other ChemotherapiesForodesineForodesine is a purine analog that inhibits purine nucleoside phosphorylase. Recruitment of patients with MF/SS with refractory disease in stages ≥IB to a phase II pivotal study was completed in 2010. The preliminary results of this multicenter study have been published recently.103 The overall response rate was around 30%. The most frequently observed adverse effects were nausea, tiredness, edema, dyspnea, and urinary calculi.
PralatrexateThe cytotoxicity induced by pralatrexate can be 10 times greater than that of methotrexate, allowing acquired tumor resistances to be overcome. The agent has been shown to be active in advanced T-cell lymphomas and it is a promising drug for maintenance therapies in CTCL.104,105
Stem Cell TransplantationAutologous stem cell transplantation can achieve high response rates but with immediate relapse (mean time to relapse, <100 days).106,107 This approach is therefore only useful in certain patients as a means to improve quality of life or as a way to buy some time before moving on to other therapies.
Long-term CR has been achieved with allogeneic stem cell transplantation, although mortality is high due mainly to graft-vs-host disease.108,109 Indeed, the efficacy of the technique is linked to the appearance of this event, known as a graft-vs-lymphoma effect,110 in which the donor lymphocytes attack the tumor lymphocytes of the receptor, thereby eliminating them. Currently, regimens are used that are not myeloablative but that are nevertheless cytoreductive prior to transplant, in an attempt to improve response and reduce complications. A recent study lends support to the role of this treatment for tumoral MF (stage IIB) and SS.111 The survival rates are significantly higher in patients with second CR, PR, or progression with 3 or fewer lines of prior systemic therapy. Administration of this therapy should therefore not be left too late in the course of the disease. In the short term, it may be the only curative treatment available.
ConclusionsCurrently, the aim in the treatment of PCL is to avoid progression to more advanced stages of the disease as, at present, there is no curative treatment available. There are no comparative studies between different systemic treatments in patients with advanced PCL, and so treatment in these patients is not at all protocolized. There is still much debate concerning the management of this disease, and so a conservative or start low go slow approach is recommended.112,113Tables 2–8 summarize the recommendations of the EORTC consensus on treatment as well as staging.20,46 The recommendations of the EORTC for treatment of CTCL should be reviewed in the light of new emerging therapies.20
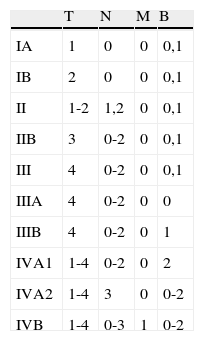
TNMB Staging for Mycosis Fungoides/Sézary Syndrome.
| Skin | |
| T1 | Limited patches, papules, and/or plaques covering <10% of the body surface area. Can be staged as T1a (only patches) vs T1b (plaques±patches) |
| T2 | Patches, papules, and/or plaques covering ≥10% of the body surface area. Can be staged as T2a (only patches) vs T2b (plaques±patches) |
| T3 | One or more tumors (≥1cm in diameter) |
| T4 | Confluence of erythema covering ≥80% of the body surface area |
| Lymph nodes | |
| N0 | No abnormal peripheral lymph nodes. Biopsy not required |
| N1 | Enlarged peripheral lymph nodes, Dutch gr 1 or NCI LN0,2 histology |
| N1a | Negative clonality |
| N1b | Positive clonality |
| N2 | Enlarged peripheral lymph nodes, Dutch gr 2 or NCI LN3 histology |
| N2a | Negative clonality |
| N2b | Positive clonality |
| N3 | Enlarged peripheral lymph nodes, Dutch gr 3-4, or NCI LN4 positive or negative clonality |
| Nx | Enlarged peripheral lymph nodes, without histologic confirmation |
| Visceral | |
| Mo | No visceral involvement |
| M1 | Visceral involvement (histologic confirmation needed and the affected organ should be specified) |
| Hematologic | |
| B0 | Absence of significant hematologic involvement ≤5% atypical lymphocytes (Sézary cells) |
| B0a | Negative clonality |
| B0b | Positive clonality |
| B1 | Low hematologic involvement: >5% peripheral lymphocytes are atypical (Sézary cells) but do not meet the criterion for B2 |
| B1a | Negative clonality |
| B1b | Positive clonality |
| B2 | Extensive hematologic involvement: ≥1000/mm3 Sézary cells with positive clonality |
In blood, Sézary cells are defined as lymphocytes with highly convoluted cerebriform nuclei. If Sézary cells cannot be used establish the tumor level B2, then 1 of the following modified criteria of the ISCL can be used along with a positive clonal rearrangement in the T-cell receptor: 1) CD4+ or C3+ expansion with a CD4/CD8 ratio of 10 or more; 2) CD4+ expansion with abnormal immunophenotype including loss of CD7 or CD26.
T-cell clonality is defined by a polymerase chain reaction analysis or southern blotting of the gene that encodes the T-cell receptor.
Abbreviations and definitions: Dutch, criteria established by the Dutch Cutaneous Lymphomas Group; ISCL, International Society of Cutaneous Lymphoma.
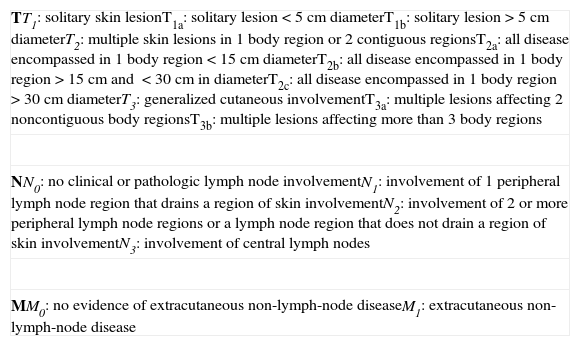
TNM Classification for Lymphomas Other Than Mycosis Fungoides/Sézary Syndrome.
| TT1: solitary skin lesionT1a: solitary lesion<5cm diameterT1b: solitary lesion>5cm diameterT2: multiple skin lesions in 1 body region or 2 contiguous regionsT2a: all disease encompassed in 1 body region<15cm diameterT2b: all disease encompassed in 1 body region >15cm and <30cm in diameterT2c: all disease encompassed in 1 body region >30cm diameterT3: generalized cutaneous involvementT3a: multiple lesions affecting 2 noncontiguous body regionsT3b: multiple lesions affecting more than 3 body regions |
| NN0: no clinical or pathologic lymph node involvementN1: involvement of 1 peripheral lymph node region that drains a region of skin involvementN2: involvement of 2 or more peripheral lymph node regions or a lymph node region that does not drain a region of skin involvementN3: involvement of central lymph nodes |
| MM0: no evidence of extracutaneous non-lymph-node diseaseM1: extracutaneous non-lymph-node disease |
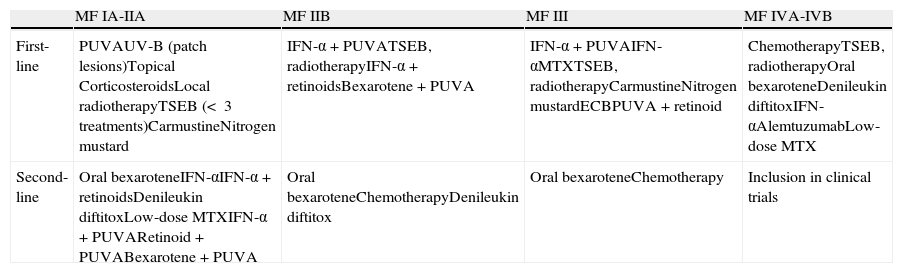
Recommendations of the European Organisation for Treatment and Research of Cancer on the Treatment of Mycosis Fungoides (MF).
| MF IA-IIA | MF IIB | MF III | MF IVA-IVB | |
| First-line | PUVAUV-B (patch lesions)Topical CorticosteroidsLocal radiotherapyTSEB (< 3 treatments)CarmustineNitrogen mustard | IFN-α+PUVATSEB, radiotherapyIFN-α+retinoidsBexarotene+PUVA | IFN-α+PUVAIFN-αMTXTSEB, radiotherapyCarmustineNitrogen mustardECBPUVA+retinoid | ChemotherapyTSEB, radiotherapyOral bexaroteneDenileukin diftitoxIFN-αAlemtuzumabLow-dose MTX |
| Second-line | Oral bexaroteneIFN-αIFN-α+retinoidsDenileukin diftitoxLow-dose MTXIFN-α+PUVARetinoid+PUVABexarotene+PUVA | Oral bexaroteneChemotherapyDenileukin diftitox | Oral bexaroteneChemotherapy | Inclusion in clinical trials |
Abbreviations: ECP, extracorporeal photophoresis; IFN-α: interferon alfa; MTX, methotrexate; PUVA, psoralen + UV-A; TSEB, total skin electron beam.
Recommendations of the European Organisation for Treatment and Research of Cancer on the Treatment of Sézary Syndrome.
| First-line |
| Extracorporeal photophoresis |
| IFN-α |
| Denileukin diftitox |
| Chlorambucil+prednisone |
| Second-line |
| Oral bexarotene |
| Chemotherapy |
| Alemtuzumab |
| MTX |
Abbreviations: IFN-α, interferon alfa; MTX, methotrexate.
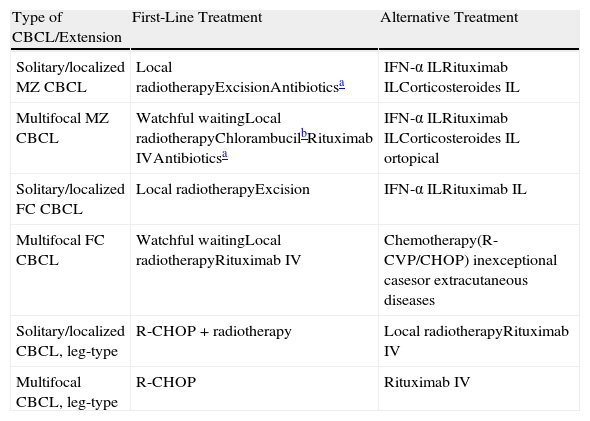
Recommendations of the European Organisation for Research and Treatment of Cancer on the Treatment of Cutaneous B-Cell Lymphoma (CBCL).
| Type of CBCL/Extension | First-Line Treatment | Alternative Treatment |
| Solitary/localized MZ CBCL | Local radiotherapyExcisionAntibioticsa | IFN-α ILRituximab ILCorticosteroides IL |
| Multifocal MZ CBCL | Watchful waitingLocal radiotherapyChlorambucilbRituximab IVAntibioticsa | IFN-α ILRituximab ILCorticosteroides IL ortopical |
| Solitary/localized FC CBCL | Local radiotherapyExcision | IFN-α ILRituximab IL |
| Multifocal FC CBCL | Watchful waitingLocal radiotherapyRituximab IV | Chemotherapy(R-CVP/CHOP) inexceptional casesor extracutaneous diseases |
| Solitary/localized CBCL, leg-type | R-CHOP+radiotherapy | Local radiotherapyRituximab IV |
| Multifocal CBCL, leg-type | R-CHOP | Rituximab IV |
Abbreviations: CHOP, cyclophosphamide, vincristine, prednisone, and doxorubicin; CVP, cyclophosphamide, vincristine, and prednisone; IFN-α, interferon alfa; IL, intralesional; IV, intravenous; MZ, mantle-zone.
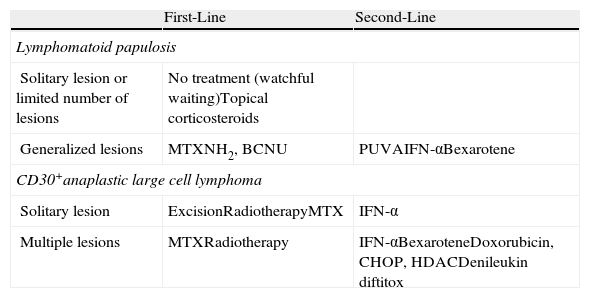
Treatment of CD30+ Cutaneous T-Cell Lymphoma (CTCL).
| First-Line | Second-Line | |
| Lymphomatoid papulosis | ||
| Solitary lesion or limited number of lesions | No treatment (watchful waiting)Topical corticosteroids | |
| Generalized lesions | MTXNH2, BCNU | PUVAIFN-αBexarotene |
| CD30+anaplastic large cell lymphoma | ||
| Solitary lesion | ExcisionRadiotherapyMTX | IFN-α |
| Multiple lesions | MTXRadiotherapy | IFN-αBexaroteneDoxorubicin, CHOP, HDACDenileukin diftitox |
Abbreviations: BCNU, carmustine; CHOP, cyclophosphamide, vincristine, prednisone, and doxorubicin; HDAC, histone deacetylase inhibitors; IFN-α, interferon alfa; MTX, methotrexate; NH2, nitrogen mustard; PUVA, psoralen + UV-A.
The authors declare that they have no conflicts of interest.
Please cite this article as: Izu-Belloso RM, García-Ruiz JC. Actualización terapéutica en linfomas cutáneos. Actas Dermosifiliogr.2012;103:694-707.