Infantile hemangiomas (IH) are a frequent vascular tumor. In recent years, propranolol has emerged as an alternative in the treatment of IH. The objective of the present study was to evaluate the effectiveness of propranolol for the treatment of IH.
Materials and methodsPatients with IH requiring treatment were included. Cardiologic evaluation was made to every patient and electrocardiogram (ECG) and echocardiogram were done.
Oral propranolol was started in an ambulatory way at a dose of 2mg/kg daily divided in two doses. At ten days all the patients were evaluated with a 24-h rhythm holter.
Evaluation of effectiveness: In clinical controls and by images IH were formally analyzed, without blindness. Response was categorized as complete response (CR), partial response (PR) and no response (NR).
Adverse events: Adverse events were registered in a special category of the formulary.
Results57 patients were included. Mean age was 9.7 months. There were 80.8% females. Mean duration of treatment was 7.3 months (1–24 months).
Efficacy: 50.6% had CR, 49.3% had PR. There were a 7% of adverse events. No differences in response rate exist according to age or location. No rhythm holter was altered at ten-day control.
ConclusionOur study highlights the possibility of starting propranolol in an ambulatory way, establishes a dose of 2mg/kg/day and confirms the security profile of the drug. We consider propranolol as a first line treatment for IH.
Los hemangiomas de la infancia (HI) son un tumor vascular frecuente. En los últimos años, el propranolol ha demostrado ser una alternativa terapéutica para los HI. El objetivo del presente estudio fue evaluar la efectividad del propranolol para los HI.
Material y métodoLo pacientes que requirieron tratamiento de sus HI fueron incluidos. Se realizó evaluación por cardiología infantil en todos los pacientes. Se inició propranolol a una dosis de 2mg/kg al día dividas en dos tomas. A los 10 días de tratamiento se realizó Holter de arritmias de 24 horas a todos los pacientes.
Evaluación de efectividad: se realizó formalmente en todos los controles clínicos y mediante iconografía, sin ciego. La respuesta se clasificó en respuesta complete (RC), respuesta parcial (RP) y no respuesta (NR). Los efectos adversos se registraron en un formulario especialmente diseñado.
ResultadosSe incluyeron 57 pacientes. La edad promedio al inicio fue de 9,7 meses. 80,8% fueron mujeres; la duración del tratamiento promedio fue de 7,3 meses (rango 1–24 meses).
Eficacia: se obtuvo 50,6% de RC y 49,3% de RP. No hubo diferencia al analizar la respuesta a tratamiento de acuerdo a la ubicación y a la edad. Hubo un 7% de eventos adversos sin haber ningún Holter alterado a los 10 días.
ConclusionesNuestro estudio destaca la posibilidad de iniciar propranolol de forma ambulatoria, establece una dosis de 2mg/kg al día y confirma el perfil de seguridad del fármaco. Nosotros consideramos propranolol el fármaco de primea línea en el tratamiento de HI; en los casos en que sea necesario el tratamiento de estas lesiones.
IH are the most common vascular tumors worldwide, affecting 5–10% of infants and up to 30% of premature babies.1 They occur four times more frequently in females than in males.2 Most of them are not present at birth and undergo rapid growing during infancy followed by an involution phase during the first few years.3
In the vast majority of patients with IH, treatment is not necessary and only strict follow-up is recommended; however, some patients with IH can be complicated and intervention is required.4 These occur when they ulcerate, have massive growth, cause cosmetic impairment or disfigurement, and if they cause impact on normal function (e.g. periorificial hemangiomas).5
Medical treatments for IH include topical therapies with corticosteroids, imiquimod or timolol6; systemic therapies with oral or intralesional glucocorticoids; chemotherapeutic agents such as interferon and vincristine; surgery, and different kinds of laser therapies and the combination of these treatments.4,5
Propranolol, a non-selective β1 and β2 antagonist developed in 1950s by the Nobel Prize Sir James Black,7 has shown to be effective in IH treatment. Since the serendipitous finding of Léauté-Labrèze et al. in 20088 many case-reports and case-series have arisen showing the usefulness of propranolol for treating IH.
Herein, we present a prospective case-series protocol of 57 IH patients treated with oral propranolol in an outpatient regimen.
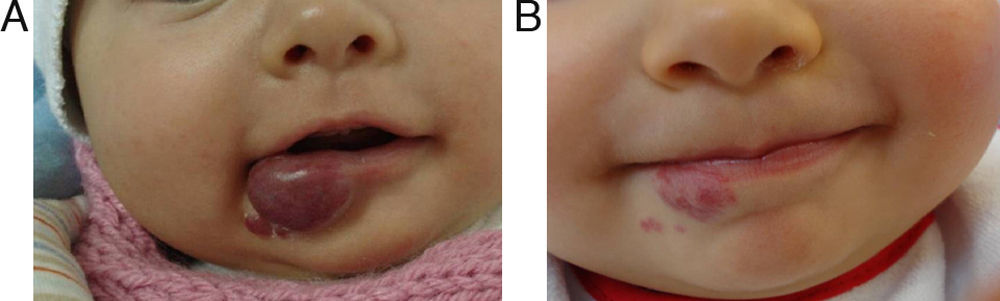
Patients and methodsHemangiomas needing treatment were defined as stated before and as commonly considered: those that may cause functional impairment or pain, ulcerated or at risk of being ulcerated, periorificial (e.g. ocular, nasal, earlobe, perioral), in the folds (e.g. axilla) and genitalia; and those who may cause scarring or disfigurement, as those in nasal tip or cheek.
This study was done between September 2008 and May 2011 at the Dermatology Department, Facultad de Medicina, Pontificia Universidad Católica de Chile.
Inclusion criteria: All infants who present at our dermatology department with IH who need treatment were offered to use propranolol. A complete history and physical examination, a basal ECG and cardiac echography (ECO) were done and all patients were sent to the pediatric cardiologist for cardiologic pass (this includes heart rate and blood pressure measurement and evaluation of exams: basal ECG and ECO). If this evaluation was normal, patients were included in the protocol. We did not request for basic biochemistry exams (glycemia, calcium, and others) unless patients were symptomatic or some disease was suspected.
Parents were told about this protocol and an instructive booklet with information about the medication was given. When there were diagnostic difficulties, an ultrasound of the involved area was done.
Exclusion criteria: Severe bradycardia, third grade atrio-ventricular blockade, or any known hypersensitivity to propranolol or cardiologic pass rejection.
Treatment protocol: All patients were received with the cardiologic pass and baseline pictures were taken. Propranolol was started in an outpatient regimen at a dose of 2mg/kg daily divided in two doses orally. Since the first dose it was given at full dose and not gradually increased. Propranolol was reformulated as 10mg per 1mL suspension. A 24-h rhythm holter was done to all patients at 10 days of treatment, if normal treatment was continued. If altered, they were sent for a new cardiology-evaluation. In this 10-day control we did not control vital signs. While in treatment, they were controlled monthly without evaluation of vital signs or biochemical exams unless they were symptomatic. We did not measure thyroid hormones in large hemangiomas. Additionally, all parents were advised of bronchial obstructive diseases and the possibility of hypoglycemia with prolonged fasting due to inherent risks of propranolol.
Patients were controlled monthly without evaluation of vital signs or glycemia.
In patients with proliferating lesions (infants under the age of 12 months) propranolol was continued until the theoretic conclusion of this proliferative phase until at least the age of 12 months. Patients who began treatment when older than 12 months took propanolol for at least 6 months. If lesions were still responding, we continued the medication. If lesions were no longer responding when the patient reached an age target, we continued treatment with the drug for 1 additional month and then discontinued it if response was not evident. If after withdrawal of propranolol lesions rebounded, we started again with the same previous scheme until achieving the same previous clinical response and then weaned as stated in the treatment protocol.
Propranolol was weaned gradually at the end of the treatment in one month to 1mg/kg daily, and then was completely stopped. If cosmetic sequels were evident at the end of the treatment, like redundant skin (that was not corrected by propranolol), patients were sent for Plastic Surgeon evaluation for surgical correction. These evaluations were not registered in this study.
Evaluation of effectiveness: All patients had baseline pictures taken before the initiation of propranolol and monthly in every control. IH were formally analyzed by images and in monthly follow-ups, without blindness, by the same investigators of the study and a global score, based on color, volume, size and consistency was entered as follows:
- 1.
CR: complete resolution of the IH. Residual lesions (telangiectasias and redundant tissue) were allowed and also considered CR.
- 2.
PR: reduction in size, change in color or consistency without being a CR.
- 3.
NR: any change at all when compared to baseline photographs or continue growing while in treatment.
This evaluation was done the last day the patients were followed-up, independently of treatment time or if treatment was already finished. We did this to evaluate the final outcome of propranolol as stated in our primary objective.
Others: Patients with ocular or periocular hemangiomas were evaluated by an ophthalmologist; if needed, an ophthalmology follow-up was indicated.
Adverse events: Adverse events were registered in a special category of the formulary. Every adverse event was consigned there only if they were symptomatic, because we did not control vital signs in follow-ups. Hypotension, bradycardia, bronchial obstruction and hypoglycemia were specially emphasized.
Informed consent: Parents of every patient signed a two-copy written informed consent included in the informative booklet. No parents rejected the use of propranolol after detailed information of the drug safety was given.
Statistical analysis: It was done using MINITAB® 15 (LEAD Technologies, Inc.). Values were analyzed using Chi-square test for age correlation and Pearson Chi-square test for analysis of location. A p-value was considered statistically significant when it was <0.05 (95%).
Results57 patients were compatible with inclusion criteria and started oral propranolol.
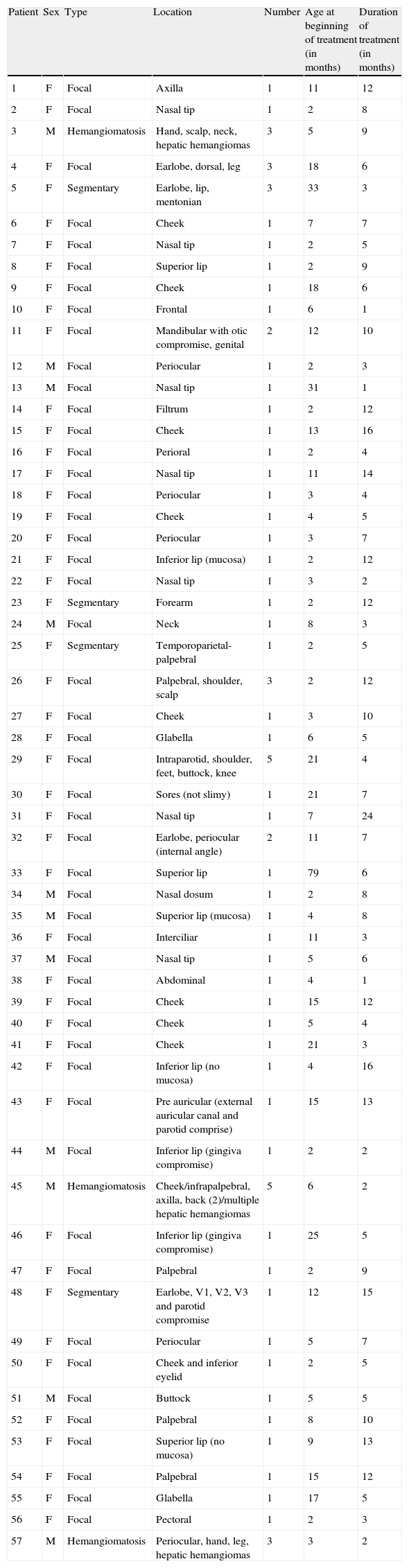
Baseline characteristics (Table 1)Mean age at the beginning of the study was 9.7 months (standard deviation 12.05, minimum 2 months, and maximum 79 months). There were 80.8% female and 19.2% male patients in the study.
Patient's characteristics and response to propranolol. IC stands for injectable corticosteroids. ASD: atrial septal defect.
| Patient | Sex | Type | Location | Number | Age at beginning of treatment (in months) | Duration of treatment (in months) |
| 1 | F | Focal | Axilla | 1 | 11 | 12 |
| 2 | F | Focal | Nasal tip | 1 | 2 | 8 |
| 3 | M | Hemangiomatosis | Hand, scalp, neck, hepatic hemangiomas | 3 | 5 | 9 |
| 4 | F | Focal | Earlobe, dorsal, leg | 3 | 18 | 6 |
| 5 | F | Segmentary | Earlobe, lip, mentonian | 3 | 33 | 3 |
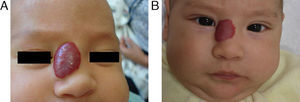
| 6 | F | Focal | Cheek | 1 | 7 | 7 |
| 7 | F | Focal | Nasal tip | 1 | 2 | 5 |
| 8 | F | Focal | Superior lip | 1 | 2 | 9 |
| 9 | F | Focal | Cheek | 1 | 18 | 6 |
| 10 | F | Focal | Frontal | 1 | 6 | 1 |
| 11 | F | Focal | Mandibular with otic compromise, genital | 2 | 12 | 10 |
| 12 | M | Focal | Periocular | 1 | 2 | 3 |
| 13 | M | Focal | Nasal tip | 1 | 31 | 1 |
| 14 | F | Focal | Filtrum | 1 | 2 | 12 |
| 15 | F | Focal | Cheek | 1 | 13 | 16 |
| 16 | F | Focal | Perioral | 1 | 2 | 4 |
| 17 | F | Focal | Nasal tip | 1 | 11 | 14 |
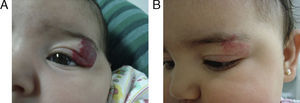
| 18 | F | Focal | Periocular | 1 | 3 | 4 |
| 19 | F | Focal | Cheek | 1 | 4 | 5 |
| 20 | F | Focal | Periocular | 1 | 3 | 7 |
| 21 | F | Focal | Inferior lip (mucosa) | 1 | 2 | 12 |
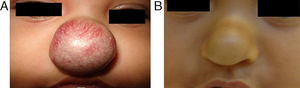
| 22 | F | Focal | Nasal tip | 1 | 3 | 2 |
| 23 | F | Segmentary | Forearm | 1 | 2 | 12 |
| 24 | M | Focal | Neck | 1 | 8 | 3 |
| 25 | F | Segmentary | Temporoparietal-palpebral | 1 | 2 | 5 |
| 26 | F | Focal | Palpebral, shoulder, scalp | 3 | 2 | 12 |
| 27 | F | Focal | Cheek | 1 | 3 | 10 |
| 28 | F | Focal | Glabella | 1 | 6 | 5 |
| 29 | F | Focal | Intraparotid, shoulder, feet, buttock, knee | 5 | 21 | 4 |
| 30 | F | Focal | Sores (not slimy) | 1 | 21 | 7 |
| 31 | F | Focal | Nasal tip | 1 | 7 | 24 |
| 32 | F | Focal | Earlobe, periocular (internal angle) | 2 | 11 | 7 |
| 33 | F | Focal | Superior lip | 1 | 79 | 6 |
| 34 | M | Focal | Nasal dosum | 1 | 2 | 8 |
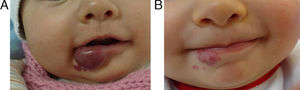
| 35 | M | Focal | Superior lip (mucosa) | 1 | 4 | 8 |
| 36 | F | Focal | Interciliar | 1 | 11 | 3 |
| 37 | M | Focal | Nasal tip | 1 | 5 | 6 |
| 38 | F | Focal | Abdominal | 1 | 4 | 1 |
| 39 | F | Focal | Cheek | 1 | 15 | 12 |
| 40 | F | Focal | Cheek | 1 | 5 | 4 |
| 41 | F | Focal | Cheek | 1 | 21 | 3 |
| 42 | F | Focal | Inferior lip (no mucosa) | 1 | 4 | 16 |
| 43 | F | Focal | Pre auricular (external auricular canal and parotid comprise) | 1 | 15 | 13 |
| 44 | M | Focal | Inferior lip (gingiva compromise) | 1 | 2 | 2 |
| 45 | M | Hemangiomatosis | Cheek/infrapalpebral, axilla, back (2)/multiple hepatic hemangiomas | 5 | 6 | 2 |
| 46 | F | Focal | Inferior lip (gingiva compromise) | 1 | 25 | 5 |
| 47 | F | Focal | Palpebral | 1 | 2 | 9 |
| 48 | F | Segmentary | Earlobe, V1, V2, V3 and parotid compromise | 1 | 12 | 15 |
| 49 | F | Focal | Periocular | 1 | 5 | 7 |
| 50 | F | Focal | Cheek and inferior eyelid | 1 | 2 | 5 |
| 51 | M | Focal | Buttock | 1 | 5 | 5 |
| 52 | F | Focal | Palpebral | 1 | 8 | 10 |
| 53 | F | Focal | Superior lip (no mucosa) | 1 | 9 | 13 |
| 54 | F | Focal | Palpebral | 1 | 15 | 12 |
| 55 | F | Focal | Glabella | 1 | 17 | 5 |
| 56 | F | Focal | Pectoral | 1 | 2 | 3 |
| 57 | M | Hemangiomatosis | Periocular, hand, leg, hepatic hemangiomas | 3 | 3 | 2 |
| Patient | Sex | Adverse events | Response | Lost of follow-up | Rebound | Previous treatment | Others |
| 1 | F | No | Partial | No | No | No | No |
| 2 | F | No | Complete | No | No | No | No |
| 3 | M | Rash | Complete | No | No | No | No |
| 4 | F | No | Partial | No | No | I.C. | No |
| 5 | F | No | Partial | No | No | I.C. | No |
| 6 | F | No | Complete | No | No | No | No |
| 7 | F | No | Partial | No | No | No | No |
| 8 | F | No | Partial | No | No | No | No |
| 9 | F | No | Partial | No | No | I.C. | No |
| 10 | F | No | Partial | No | No | No | No |
| 11 | F | No | Complete | No | No | I.C. | No |
| 12 | M | Overdose | Partial | No | No | No | No |
| 13 | M | No | Partial | Yes | No | I.C. | No |
| 14 | F | No | Complete | No | No | No | No |
| 15 | F | No | Complete | Yes | No | No | No |
| 16 | F | No | Complete | No | No | No | No |
| 17 | F | No | Complete | No | Yes | No | No |
| 18 | F | No | Partial | No | No | No | No |
| 19 | F | No | Partial | No | No | No | No |
| 20 | F | No | Partial | No | No | No | No |
| 21 | F | No | Partial | No | No | No | No |
| 22 | F | No | Complete | Yes | No | No | No |
| 23 | F | No | Complete | No | No | No | No |
| 24 | M | No | Partial | No | No | No | No |
| 25 | F | No | Complete | No | No | No | No |
| 26 | F | No | Complete | No | No | No | Left hemiblock |
| 27 | F | No | Complete | No | Yes | No | No |
| 28 | F | No | Complete | No | Yes | No | Persistent ductus arteriosus |
| 29 | F | No | Complete | No | No | No | No |
| 30 | F | No | Partial | No | No | I.C. | No |
| 31 | F | No | Partial | No | Yes | I.C. | No |
| 32 | F | No | Complete | No | No | No | Osteogenesis imperfecta |
| 33 | F | No | Partial | No | No | I.C. | No |
| 34 | M | Sudoration | Partial | No | No | No | No |
| 35 | M | No | Complete | No | No | No | Ventricular extrasystole |
| 36 | F | No | Complete | Yes | No | No | No |
| 37 | M | No | Complete | Yes | No | No | No |
| 38 | F | No | Partial | Yes | No | No | No |
| 39 | F | No | Complete | No | No | No | No |
| 40 | F | No | Partial | No | No | No | ASD/apneas |
| 41 | F | No | Complete | Yes | No | No | No |
| 42 | F | No | Partial | No | No | No | No |
| 43 | F | No | Partial | No | Yes | I.C. | No |
| 44 | M | No | Partial | No | No | No | No |
| 45 | M | Sleepiness | Partial | Yes | No | No | Persistent ductus and bronchopulmonar dysplasia |
| 46 | F | No | Complete | No | No | No | No |
| 47 | F | No | Complete | No | No | No | No |
| 48 | F | No | Partial | No | Yes | No | No |
| 49 | F | No | Partial | No | No | No | No |
| 50 | F | No | Complete | No | No | No | No |
| 51 | M | No | Partial | No | No | No | No |
| 52 | F | No | Complete | No | No | No | No |
| 53 | F | No | Complete | No | No | No | No |
| 54 | F | No | Complete | No | Yes | No | No |
| 55 | F | No | Complete | No | No | No | No |
| 56 | F | No | Complete | No | No | No | Persistent ductus arteriosus |
| 57 | M | No | Partial | No | No | No | No |
There were 77 hemangiomas, mean number of hemangiomas per patient was 1.3 (standard deviation 0.9). 9 of 57 patients had more than one IH; two patients had five hemangiomas. 87.7% of patients had focal hemangiomas and 5.2% had multiple hemangiomas (defined as more than five IH and visceral hemangiomas).
7% of patients had segmentary IH; of them, 3 patients were on trigeminal branches distribution (patients 5, 25 and 48) that prompted a study for PHACE syndrome. The study was done in an ambulatory way with magnetic resonance of brain and neck with angiography. ECO was not done because it was requested in the preliminary study. They were also evaluated by an ophthalmologist. In the three of them the study was normal and could use propranolol.
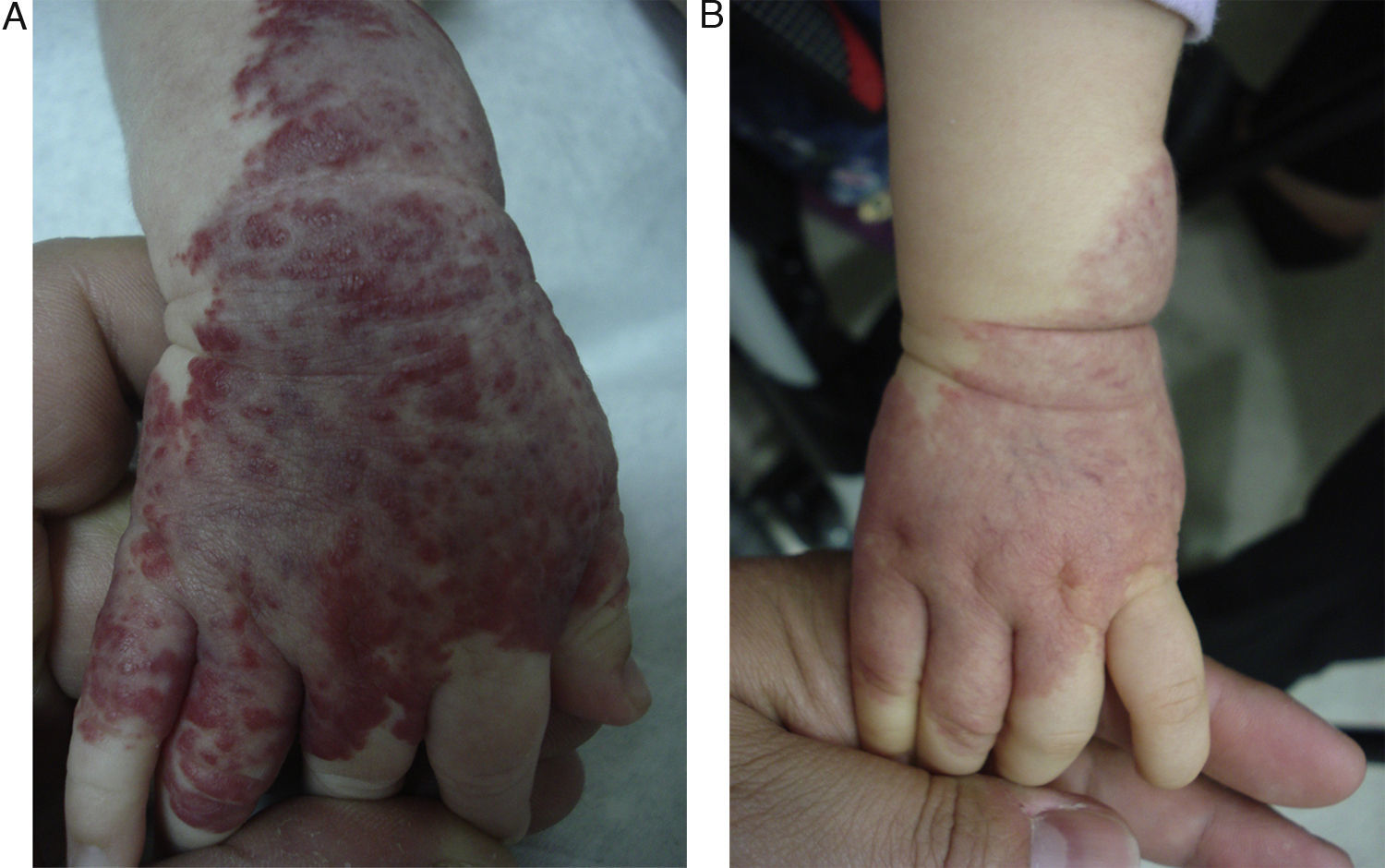
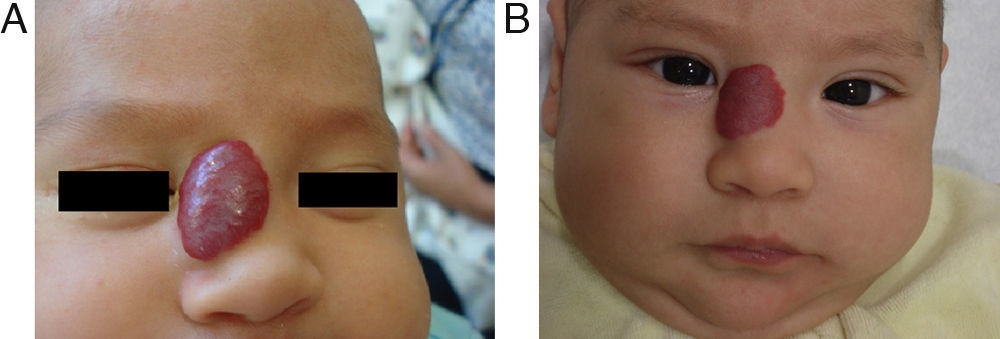
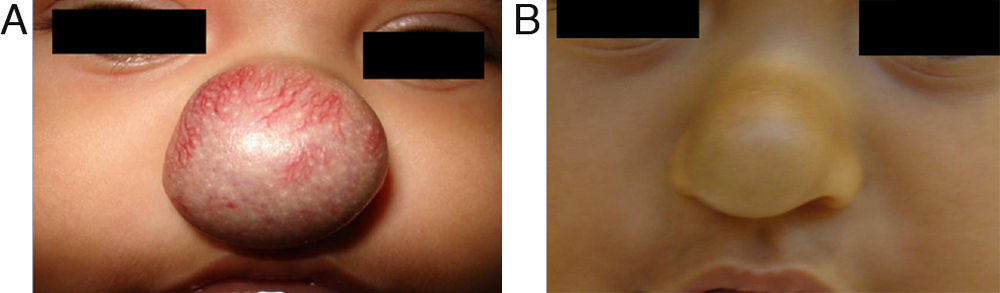
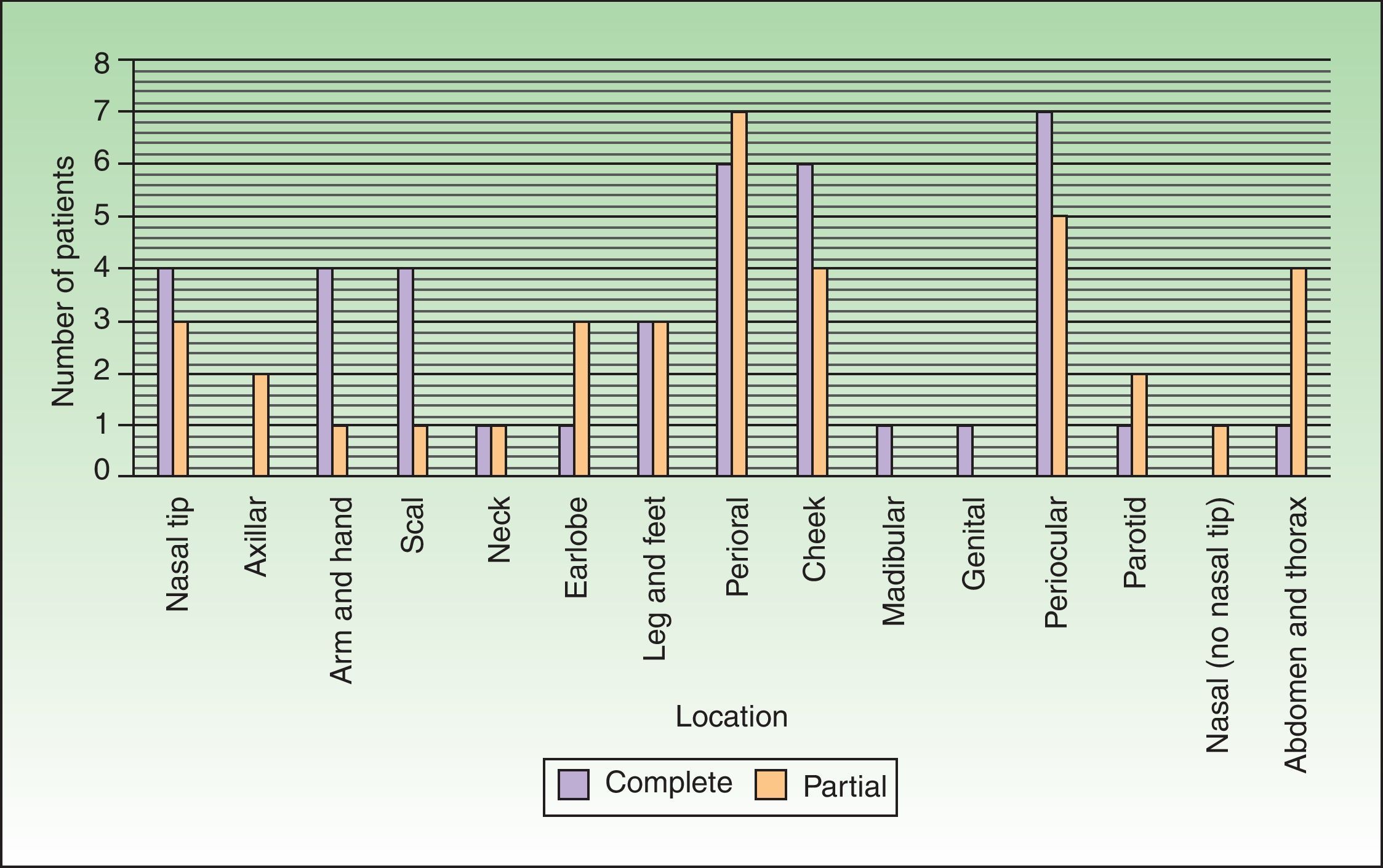
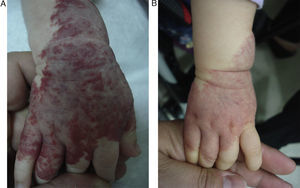
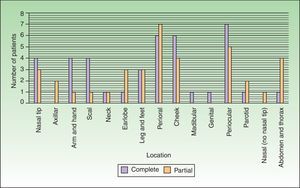
Hemangiomas by site were as follows: 58 of 77 hemangiomas (75.4%) were on head and neck (twelve periocular, ten on lips, nine on cheeks, six were on nasal tip, five on the scalp, four on earlobe, two on parotid region, two on neck, one on mandible, one on filtrum, one interciliary, one segmentary on trigeminal branch V1, V2 and V3, one preauricular, one in nasal dorsum, one on glabella and one on chin region). Two were on axilla; five were on shoulder; forearm or hand; six were on lower limbs; five were on trunk and there was one genitalia involving hemangioma. There were three patients with visceral hemangiomas; all of them were multiple hepatic hemangiomas and were not considered in the total number of IH.
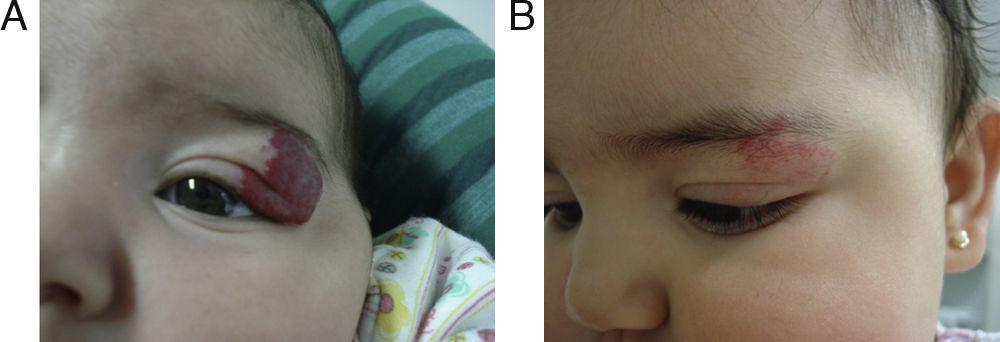
Patients with ocular or periocular involvement were evaluated by a pediatric ophthalmologist. All the evaluations were normal and a follow-up was scheduled in 6 months. Patient 25 had almost complete occlusion that led to the development of amblyopia. She started treatment with an occlusive dressing 4h daily to the healthy eye and a monthly control was started. She improved notably with propranolol. This patient is still on follow-up because of persistent amblyopia.
Four patients had mucosal involvement. We did not consider mucosal involvement in one patient who had involvement of the ear canal because his IH did not compromise tympanic mucosa. Three patients had parotid involvement.
At the beginning four patients had ulcerated IH. Patients 14, 25 and 45 started propranolol and mupirocin 2% twice daily for 7 days and at 1-month follow-up ulceration was not evident and completely healed. Patient 51 was treated with topical chloramphenicol and 4 sessions of pulse-dye laser in another center with PR and an incomplete healing of the ulceration. Propranolol was started and at one-month follow-up it was completely healed.
Nine patients have had previous treatments, of them; all used intralesional corticosteroids (IC). In five of them parents did not remember the drug or dose used and they were treated in other center but all have PR or NR. Patient 4 used 10mg triamcinolone monthly for 4 months since 3 months with PR; patient 9 used 10mg triamcinolone monthly for 5 months since 9 months with PR. Patients 11 and 13 both used betamethasone (dose not reported) monthly for 6 months since 3 months of age with NR. Patient 30 used 10mg triamcinolone monthly for 3 months since 5 months with PR.
EfficacyPatients were evaluated monthly with the protocol stated earlier; some of them had finished their treatment while others were still on treatment when this paper was written. We considered the last visit for evaluating effectiveness and as the final outcome. 50.6% (39/77) of IH had CR evaluated by the three investigators. 49.3% (38/77) had PR, and 0/77 IH had NR. Additionally, no IH continued growing while in treatment (Figs. 1–5). At one-month follow-up, one patient with hepatic hemangiomas had an abdominal ultrasound showing decrease in size (from 1.6cm to 1cm).
Treatment duration had a mean of 7.3 months (standard deviation 4.6) with a minimum of 1 and a maximum of 24 months. Nine patients were lost at follow-up so definitive analysis could not be made and the efficacy of response was that of their last control.
Response rate underwent a subgroup analysis. In those 7 months or older CR rate was 57.1% and in those 6 months or younger it was 45%. These differences were not statistically significant (p-value: 0.29). Also, we did not find any statistically significant differences according to IH location (p-value: 0.55) or type (focal or segmentary; p-value: 0.93) (Fig. 6).
When propranolol was withdrawn 7.7% of IH rebounded. Patient 17 was treated for six months and after 3 months without propranolol the lesion rebounded; she was then treated for an additional period of 8 months and after 4 months of follow-up since withdrawal, the IH was still in CR. Patient 28 used propranolol for 5 months and had a little rebound after 1 month who did not require restart of treatment.
Patient 31 was treated for 7 months. The IH grew again after 3 months without propranolol and was treated again for 12 months and then withdrawn. After 15 days it rebounded again so propranolol was held for a total of 24 months. Patient 43 was treated for 8 months; then she was 4 months without treatment and her IH rebounded so propranolol was restarted for 5 additional months.
Patient 48 had a parotid focal IH that was treated for 10 months and treatment was suspended; after 5 months without treatment the IH reappeared so propranolol was restarted for 5 months and is still in treatment. Patient 54 completed 12 months of treatment; after 2 months without the drug parents referred a rebound but propranolol was not restarted.
In all these cases when propranolol was restarted the response rate, which was reached within the prior treatment protocol, was recovered. The drug was then stopped as stated in the protocol in ‘Patients and methods’ section.
Adverse eventsNo 24-h rhythm holter was altered at ten-day follow-up.
There were 4 adverse events in our register (7% of patients). One patient with sweating (glycemia 91mg/dL; no control of vital signs) resolved spontaneously. One patient was with somnolence at third day of treatment: he consulted at emergency room with normal glycemia (104mg/dL), normal blood pressure (120/60mmHg) and with normal heart rate (140beats/min). He was sent home and also resolved spontaneously. One patient had a skin rash (that responded to anti-histamines).
Patient 12 had an overdose because of dose mistake by his mother (ten times the dose). She gave 0.3mL instead of 3mL (20mg/kg). In the emergency room he was restless, euphoric and with insomnia. Blood pressure, heart rate and glycemia were normal. This last patient needed 24h hospitalization in an intensive care unit for motorization and did not contraindicate the use of propranolol.
15/57 (26.3%) of the patients had lower respiratory tract infection (bronchitis) that were highly probable in the context of a viral infection and prompted an eventual withdrawal of the drug. They were not considered an adverse event of the drug in any patient.
Others7/57 patients had comorbidities known by the parents before the initiation of treatment that were not influenced nor contraindicated propranolol: one patient had osteogenesis imperfecta, one patient had ventricular extra-systole while in treatment, one patient had left bundle hemi-block, one patient had atrial septal defect and apneas, three patients had patent ductus arteriosus (one also had bronchopulmonar dysplasia). Treatment did not influence any of these patients and follow-up was done similar to patients without comorbidities, after the primary evaluation with a cardiologist.
DiscussionWe have presented 57 patients with IH treated with propranolol; to our knowledge, one of the largest series published in English and Spanish literature.5,9–15 Since the extraordinary response of IH published in 2008,8 our group started a protocol for propranolol in treating IH.
In our series, we found a 50.6% of CR, which is a little bit lower than published elsewhere5,9–16 but similar to the 66.6% of CR reported by Bernabeu-Wittel et al.13 Sans et al. found a 100% of response on 32 children with IH.11 Buckmiller et al.5 reported that 97% of their 32 patients had any response to propranolol. Bagazgoitia et al. found an average reduction of IH of 60% evaluated as a score considering size and color as the main features.12
These differences could be explained because we considered CR as the main outcome, which is a more standardized way for the evaluation and interpretation of the response rate of IH to propranolol than “any response” used in some reports. If we had considered the final outcome as “any response”, we would have found a response rate of 100%, with any patient falling into the category of NR.
The oldest infant treated with propranolol in the literature is also in our series: a 79-month-old female who was previously treated with intralesional corticosteroids with PR; she completed 6 months of treatment with propranolol with good response.
Under our results, it seems that there is no difference in propranolol response in infants younger or older than six months of age when starting the drug. This was also found in other series.12,13 Also, we did not find any statistically significant association between IH location or type (focal or segmentary) and response rate; however the number of patients treated in the location groups is low for a definite statistical analysis; however, this results are similar to other IH series12,13 and are in concordance with our feelings that IH respond to propranolol in any location or any type of IH.12 We cannot deny that differences according to hemangiomas location or type may be found if larger series analyze this association in future studies.
An additional feature of our study is that seven patients had cardiologic and respiratory comorbidities prior to initiation of treatment that did not contraindicate propranolol and did not produce any symptomatic adverse events on patients. This is a new insight on the use and safety profile of this drug in patients with cardiopulmonary comorbidities without any adverse events and without the need for strict monitoring in addition to the first cardiologic visit. This opens a window of possibilities to children that would be automatically excluded of the opportunity to receive propranolol because of these cardio-respiratory comorbidities. We encourage an evaluation with a pediatric cardiologist to balance the risk–benefit ratio in these patients.
Recently some case series of ulcerated hemangiomas responding to propranolol have been published,12,14–16 however, variable results exist.13 We found four patients with ulcerated IH, and all of them healed in the first month of treatment. Also, one patient had NR to multiple treatments before the start of propranolol. This is a very important feature because ulcerated hemangiomas are often difficult to treat, they are painful, they can prompt to locally or systemic infections and cause scars and disfiguration and therefore always require treatment; making propranolol a good alternative for ulcerated IH as commented by other authors.12–16
We only found four adverse events; one was a serious adverse event but it was because the patient's parents did not understand our indications so they gave him ten times the dose. He needed 24h-transient hospitalization with cardiorespiratory monitoring in an intensive-care unit but he did not have bradycardia, hypotension or hypoglycemia; so no serious adverse events were found if propranolol is well administered without overdosing. The others were just mild adverse events. Similar adverse events profiles have been reported by Manunza et al.9 and commented by other authors.17
Other authors have observed more severe adverse events; de Graaf et al.18 found two patients with hypoglycemia. One of them had adrenal insufficiency secondary to a sudden withdrawal of corticosteroids and the other had prolonged fasting when presenting it. They also found bronchial hyperresponsiveness, hypotension, restless sleep, constipation and cold extremities. Others had shown similar adverse events: increased sleepiness, gastro-esophageal reflux, allergic rash and respiratory virus exacerbation.5 Other adverse events reported are masking of the initial clinical signs of cardiac failure and diminish of cardiac performance; lessening clinical features of hypoglycemia19 and hypoglycemia20 particularly in patients younger than 1 year,21 hyperkalemia, maybe mediated by tumoral lysis mechanism,22 bradycardia,23 diarrhea,24 and probable cerebral hypoperfusion and theoretical brain tissue infarction in patients with PHACES syndrome23; this hypothesis is not accepted by all and no cerebral infarction has been found in propranolol treated patients.9 We did not find any patient with nightmares or agitated dreams, a feature reported in 14% of patients by other authors.12
Propranolol has also been successful for hepatic hemangiomas25 and airway IH.26 Three patients of our series had hepatic hemangiomas, two of them were asymptomatic and we did not perform hepatic images after treatment. One patient had a control abdominal ultrasound showing a decrease in size of hepatic hemangiomas.
One flaw of our study is the absence of a control group to compare outcomes; another weakness is the absence of an objective way to measure the improvement of IH; we tried to make an objective measurement but it is not as objective as it should be; we intended to by-pass this with an independent evaluation by three investigators of the study (MSZ, CN, AA) attempting to isolate the “complete response”13 instead of the previously reported “any response”. We cannot fully ensure that some IH classified as “complete response” are really a “partial response”.
This study is different from others published elsewhere because we started propranolol in an ambulatory way, with an initial dose of 2mg/kg/day without making an escalating dose, and without strict monitoring of vital signs in the first hours or in every control. We found this simpler scheme safe and without any serious adverse events on 57 patients and almost three years of experience. Patients were given the treatment (after cardiologic pass) and controlled 10 days later with a 24-h rhythm holter; then they were cited every month. As none of the rhythm holters made to our patients were abnormal; we suggest that this costly and troublesome exam should not be done.
We neither measured serum chemistry nor controlled vital signs in monthly follow-ups. We only referred patients to the emergency department if they were symptomatic for evaluation of heart rate, blood pressure, pulse oximetry and glycemia. Doing this, we enhanced specificity but sacrifice sensitivity. We also think that these measures may lower costs to the health systems and to the patients.
In view of our results, and what is published elsewhere about the safety profile of propranolol and beta-blockers in children,27 we postulate that basal echocardiogram and 24-h rhythm holter at 10 days should not be done and we disagree with starting propranolol in an inpatient way. We also encourage an initial cardiologic visit for a complete cardiologic physical examination; our group suggests these procedures as being sufficient to start the drug in an outpatient way, as outlined by López Gutiérrez.28 Parents must be reaffirmed about the dosage of propranolol because we found that the only severe adverse event was originated in a propranolol-overdose. We prescribe the drug two times per day instead of three times per day to increase adherence to treatment,29,30 an important issue to asses when giving any treatment. We believe that less doses is a better compliance.
We agree with some authors31,32 that propranolol must be the first-line treatment for IH because it is highly efficient and with a low adverse-events profile. We also suggest that the dose of propranolol for treating IH is 2mg/kg/day divided in two daily doses17 to increase adherence to treatment.
Conflicts of interestThe authors declare that they have no conflicts of interest.