Hyperhidrosis is very common and has a considerable impact on patients’ quality of life. While oral oxybutynin is associated with good response rates, adverse effects are common and frequently cause patients to stop treatment. Following the recent launch of oxybutynin in a transdermal patch formulation in Spain, we undertook a preliminary study to assess treatment response and adverse effects in patients with hyperhidrosis.
Material and methodsThis prospective study of 25 patients treated twice weekly with transdermal oxybutynin patches over 10 weeks assessed treatment response on 2 subjective scales: the Hyperhidrosis Disease Severity Scale (HDSS) and a visual analog scale (VAS) for sweating.
ResultsSixty percent of patients showed an improvement in HDSS scores. VAS scores improved in all cases, and 68% of patients achieved a reduction of 3 points or more. Just 2 patients (8%) experienced treatment-related adverse effects (irritant dermatitis at the patch application site in both cases).
ConclusionsAlthough our results are based on a small sample, they suggest that transdermal oxybutynin could be a useful option for the treatment of hyperhidrosis and that it has an excellent safety and tolerability profile.
La hiperhidrosis (HH) es una condición muy prevalente que supone una repercusión importante en la calidad de vida. Entre las opciones terapéuticas disponibles, la oxibutinina oral consigue buenas tasas de respuesta aunque con frecuentes efectos secundarios que condicionan en muchas ocasiones el abandono del tratamiento. Tras la comercialización en nuestro país de la oxibutinina en presentación transdérmica, realizamos un estudio preliminar para valorar el control de la HH y el perfil de efectos secundarios de este tratamiento.
Material y métodosSe realizó un estudio prospectivo con 25 pacientes que recibieron tratamiento con 2 parches semanales de oxibutinina transdérmica durante 10 semanas. La respuesta terapéutica se valoró mediante 2 escalas subjetivas: Hyperhidrosis Disease Severity Scale (HDSS) y escala analógica visual (EAV).
ResultadosUn 60% de los pacientes consiguieron una reducción en la puntuación de la HDSS. Todos los casos obtuvieron una disminución en la puntuación de la EAV, siendo esta de 3 puntos o superior en el 68% de los pacientes. Solo 2 pacientes (8%) presentaron efectos adversos relacionados con el tratamiento, en ambos casos en forma de dermatitis irritativa en la zona de aplicación del parche.
ConclusionesAunque se trata de una experiencia limitada, los resultados de nuestro estudio sugieren que la oxibutinina transdérmica podría tener utilidad en el manejo de la HH, con un excelente perfil de seguridad y tolerancia.
Hyperhidrosis (HH) is a condition in which the patient sweats more than is necessary to regulate body temperature. HH can be classed as primary and secondary. In primary HH (PHH), there is no underlying disease that accounts for the excessive sweating, whereas in the secondary form, excessive sweating can be attributed to various causes (eg, abnormalities of the endocrine and nervous systems, cancer, and treatment with drugs).1
PHH is defined as the presence of excessive sweating for at least 6 months with no justifiable cause (major criterion) and 2 or more of the following minor criteria: (1)bilateral and symmetrical distribution; (2)at least 1 episode of excessive sweating per week; (3)interference with daily activities; (4)cessation of sweating during sleep; (5)onset before age 25 years; and (6)family history of PHH.2 The most frequently involved sites are the axillas and palms, followed by the soles and facial region. PHH often affects various sites in the same patient (multifocal) and may even be generalized.3
PHH is thought to affect approximately 2%-3% of the population, and even though it is not considered a severe disease, its effect on the patient's quality of life is very significant. According to various quality of life rating scales, its effect is equivalent to that of much more severe conditions, such as diabetes.4
PHH can be managed using various therapeutic options, including drugs (topical and systemic), nonsurgical interventions (eg, iontophoresis and botulinum toxin), and surgery (selective sympathectomy and excisional surgery).5
Oxybutynin is an anticholinergic drug whose efficacy in the control of PHH is well-documented, although its use in this disease is off-label.6–9 It is important to note that none of the systemic options used for PHH are currently authorized for treatment of the disease in Spain. HH responds well to treatment with oral oxybutynin; however, adverse effects are common (mainly dry mouth and throat), prove uncomfortable for the patient, and can lead to discontinuation of treatment.
In 2014, a transdermal patch formulation of oxybutynin was marketed in Spain. According to the summary of product characteristics, it was indicated for the treatment of overactive bladder (as with the oral formulation). The transdermal patch avoids the first stage of liver metabolism, thus—in theory—reducing adverse effects and the possibility of drug interactions.10
Our hypothesis was that PHH could be improved by minimizing the adverse effects of oral oxybutynin. Therefore, we proposed a pilot study in which we applied transdermal oxybutynin patches in patients with PHH. The primary objective of this study was to evaluate the effectiveness of the transdermal patch; the secondary objective was to describe its tolerance profile.
Material and MethodsWe performed a prospective study from February to September 2015. We included 25 patients whose PHH could not be suitably controlled with topical treatments and excluded patients who had received botulinum toxin during the previous 2 years or who had undergone surgery for PHH (selective sympathectomy or excisional surgery). All patients were given exhaustive information about the transdermal oxybutynin patch and alternative treatments and about the conditions of the study. They all gave their written informed consent. The effectiveness of treatment was measured using 2 subjective scales: a visual analog scale (VAS), in which sweating was graded from 1 to 10 (with 10 as the maximum degree of sweating and 1 the minimum); and the validated Hyperhidrosis Disease Severity Scale (HDSS)11 (Table 1).
Hyperhidrosis Disease Severity Scale.
| Score | Patient's Response |
|---|---|
| 1 | My sweating is never noticeable and never interferes with my daily activities |
| 2 | My sweating is tolerable but sometimes interferes with my daily activities |
| 3 | My sweating is barely tolerable and frequently interferes with my daily activities |
| 4 | My sweating is intolerable and always interferes with my daily activities |
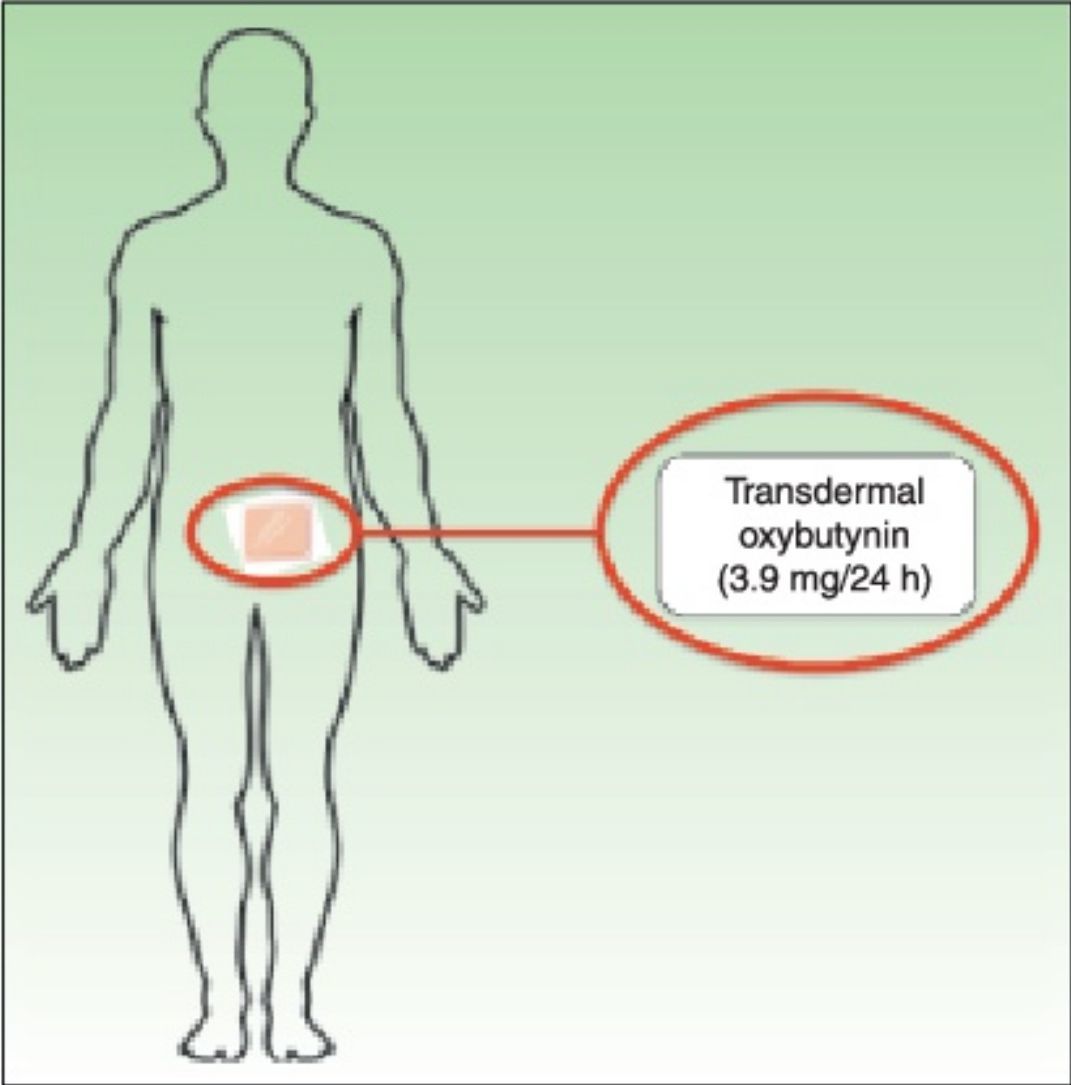
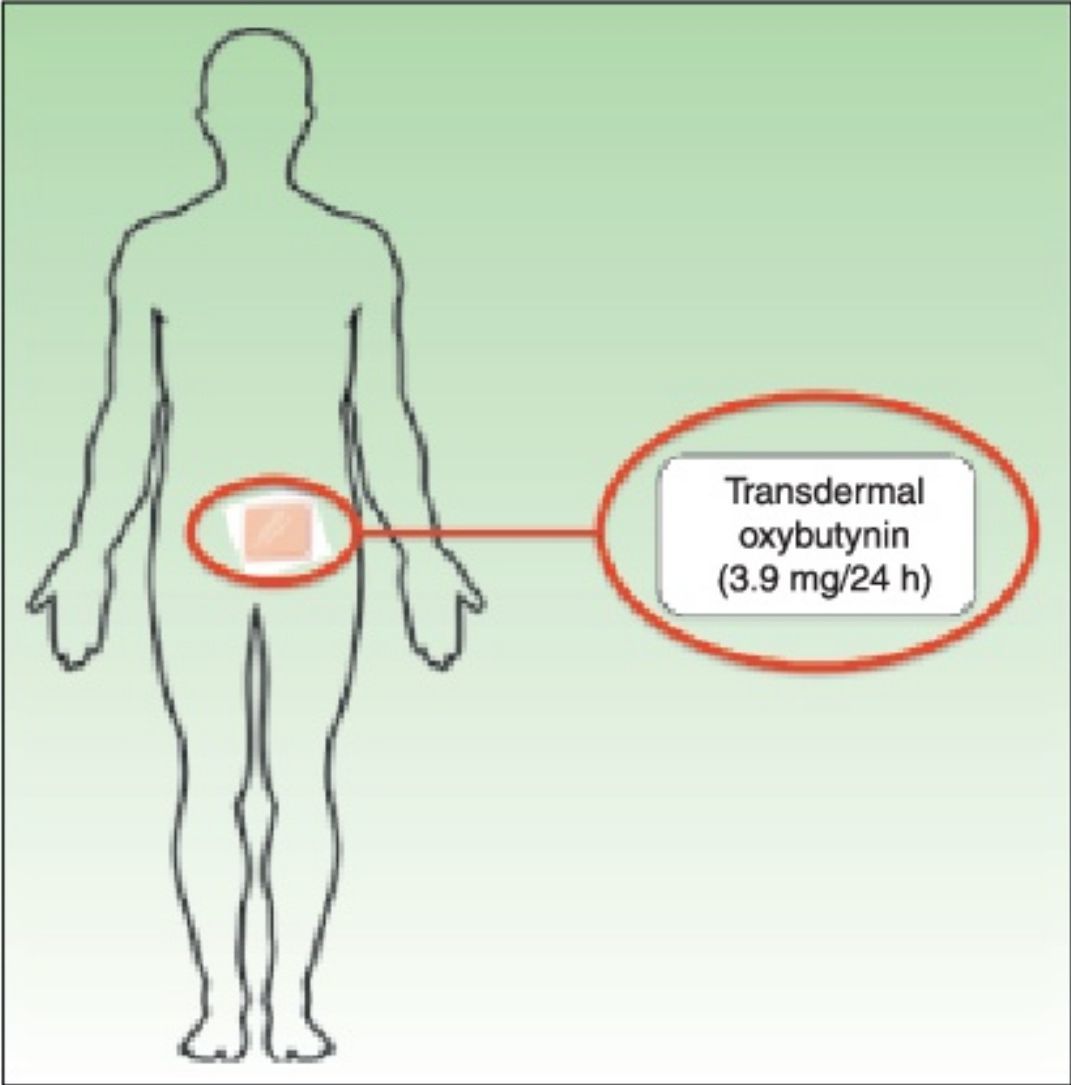
Treatment with transdermal oxybutynin patches (Kentera, Gebro Pharma) was administered for 10 weeks. Patients applied 2 patches (36mg each) per week, rotating between the thighs, hips, and abdomen. The interval of 10 weeks was based on findings for long-term oral oxybutynin, in which a lack of response at 6 weeks of treatment is considered therapeutic failure.6
The study comprised 3 visits. At the initial visit (visit1), patients were informed about the study and gave their informed consent, a baseline evaluation of HH was made using the VAS and HDSS, and treatment was started at the dose described above. Visit 2 was 6 weeks after the start of treatment and visit 3 at 10 weeks. Treatment was maintained until visit 3. The HDSS and VAS were applied at visits 2 and 3, and any adverse effects were recorded. At visit3, patients evaluated how well treatment had controlled their disease, classifying the outcome as no improvement, slight nonsignificant improvement, or significant improvement.
The demographic characteristics of the patients included in the study and the sites affected by PHH are shown in Table 2.
Demographic Characteristics and Outcome.
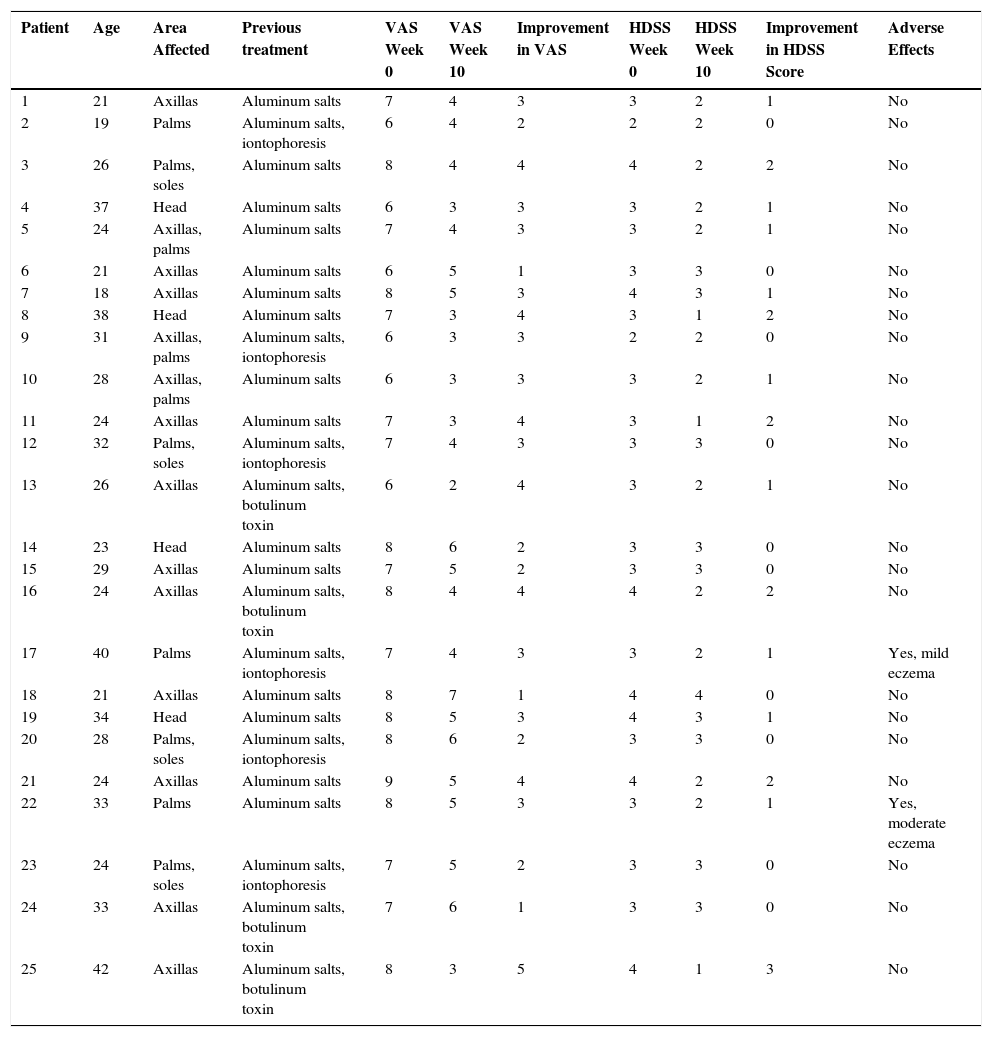
| Patient | Age | Area Affected | Previous treatment | VAS Week 0 | VAS Week 10 | Improvement in VAS | HDSS Week 0 | HDSS Week 10 | Improvement in HDSS Score | Adverse Effects |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | Axillas | Aluminum salts | 7 | 4 | 3 | 3 | 2 | 1 | No |
| 2 | 19 | Palms | Aluminum salts, iontophoresis | 6 | 4 | 2 | 2 | 2 | 0 | No |
| 3 | 26 | Palms, soles | Aluminum salts | 8 | 4 | 4 | 4 | 2 | 2 | No |
| 4 | 37 | Head | Aluminum salts | 6 | 3 | 3 | 3 | 2 | 1 | No |
| 5 | 24 | Axillas, palms | Aluminum salts | 7 | 4 | 3 | 3 | 2 | 1 | No |
| 6 | 21 | Axillas | Aluminum salts | 6 | 5 | 1 | 3 | 3 | 0 | No |
| 7 | 18 | Axillas | Aluminum salts | 8 | 5 | 3 | 4 | 3 | 1 | No |
| 8 | 38 | Head | Aluminum salts | 7 | 3 | 4 | 3 | 1 | 2 | No |
| 9 | 31 | Axillas, palms | Aluminum salts, iontophoresis | 6 | 3 | 3 | 2 | 2 | 0 | No |
| 10 | 28 | Axillas, palms | Aluminum salts | 6 | 3 | 3 | 3 | 2 | 1 | No |
| 11 | 24 | Axillas | Aluminum salts | 7 | 3 | 4 | 3 | 1 | 2 | No |
| 12 | 32 | Palms, soles | Aluminum salts, iontophoresis | 7 | 4 | 3 | 3 | 3 | 0 | No |
| 13 | 26 | Axillas | Aluminum salts, botulinum toxin | 6 | 2 | 4 | 3 | 2 | 1 | No |
| 14 | 23 | Head | Aluminum salts | 8 | 6 | 2 | 3 | 3 | 0 | No |
| 15 | 29 | Axillas | Aluminum salts | 7 | 5 | 2 | 3 | 3 | 0 | No |
| 16 | 24 | Axillas | Aluminum salts, botulinum toxin | 8 | 4 | 4 | 4 | 2 | 2 | No |
| 17 | 40 | Palms | Aluminum salts, iontophoresis | 7 | 4 | 3 | 3 | 2 | 1 | Yes, mild eczema |
| 18 | 21 | Axillas | Aluminum salts | 8 | 7 | 1 | 4 | 4 | 0 | No |
| 19 | 34 | Head | Aluminum salts | 8 | 5 | 3 | 4 | 3 | 1 | No |
| 20 | 28 | Palms, soles | Aluminum salts, iontophoresis | 8 | 6 | 2 | 3 | 3 | 0 | No |
| 21 | 24 | Axillas | Aluminum salts | 9 | 5 | 4 | 4 | 2 | 2 | No |
| 22 | 33 | Palms | Aluminum salts | 8 | 5 | 3 | 3 | 2 | 1 | Yes, moderate eczema |
| 23 | 24 | Palms, soles | Aluminum salts, iontophoresis | 7 | 5 | 2 | 3 | 3 | 0 | No |
| 24 | 33 | Axillas | Aluminum salts, botulinum toxin | 7 | 6 | 1 | 3 | 3 | 0 | No |
| 25 | 42 | Axillas | Aluminum salts, botulinum toxin | 8 | 3 | 5 | 4 | 1 | 3 | No |
Abbreviations: HDSS, Hyperhidrosis Disease Severity Scale; VAS, visual analog scale.
We included 25 patients (16 women and 9 men) with a mean age of 28years (18-42 years). They had all received treatment for HH (Table 2).
Control of symptoms was evaluated using the VAS and HDSS after 10 weeks of treatment (Table 2). Symptoms did not worsen in any patients during the study.
The VAS score decreased for all patients (1 point in 3 patients, 2 points in 5 patients, 3 points in 10 patients, 4 points in 6 patients, and 5 points in 1 patient).
The HDSS score decreased by 3 points in 1 patient, 2 points in 5 patients, and 1 point in 9 patients; the HDSS score remained unchanged in 10 patients after treatment.
No variations in the scores for these scales were recorded between week 6 (visit2) and week 10 (visit3).
As for the general evaluation at visit 3, no patients defined the response as no improvement, 8 patients (32%) considered it a slight nonsignificant improvement, and 17 (68%) patients considered it a significant improvement.
Adverse effects were reported in 2 patients (8%). In both cases, the adverse effect was irritation at the site where the patch was placed. This was classed as mild (erythema and desquamation) in patient 17 and moderate (erythema, edema, and desquamation) in patient 22. The adverse effect did not lead either of the 2 patients to discontinue treatment, since it was controlled by applying the patch at another site (patient 17) and with application of low-potency topical corticosteroids (patient 22).
DiscussionThe basic function of sweating is to regulate body temperature in response to various stimuli, such as heat and exercise. The sweat glands are activated by the sympathetic nervous system: thermoregulatory signals from the hypothalamus are transmitted via sympathetic preganglionic and postganglionic nerve endings to the sweat glands. The main neurotransmitter involved in this process is acetylcholine, which acts via the nicotinic receptors (preganglionic and postganglionic fibers) and muscarinic receptors (sweat glands). PHH is the result of an autonomic nervous system dysfunction without morphologic or quantitative abnormalities in the sweat glands.5
No therapeutic algorithm has been agreed upon for management of PHH, and the best option should be decided on a case-by-case basis. Therefore, it is important to take into account the individual characteristics of the patient and disease, the safety profile of the various treatments, and the resources available and their cost, starting with the least expensive treatments and/or treatments with fewer potential adverse effects. Consequently, the approaches considered first-line are topical products that act directly on the sweat gland, mainly aluminum salts.12
Topical anticholinergic drugs, mainly glycopyrrolate, have also been used.13–16 This active ingredient is no longer marketed in Spain, although it can be obtained as a foreign medication. Glycopyrrolate has been used at various concentrations (0.5%-4%) and in various vehicles (solution, gel, and cream), with results that vary according to the location of PHH and the concentration of the active ingredient.17 Adverse effects resulting from systemic absorption of the drug have been reported when it is used on more extensive areas of the body.18
Oxybutynin is marketed abroad as a topical formulation (gel) for the treatment of bladder conditions, although there are no references in the literature on the use of this formulation for the management of PHH.
Of all the systemic options, the best results have been achieved with anticholinergic drugs, and broad experience has been accumulated with oral methantheline bromide, oxybutynin, and glycopyrrolate. Most studies have been performed with oxybutynin.10,11,13,14
Oral oxybutynin is metabolized in the liver by isoenzyme CYP3A4 of cytochrome P450; therefore, it can react with other drugs that inhibit this system (eg, azole antifungal agents and macrolides). Its main limitation in the treatment of PHH is the frequency of adverse effects, which, although not generally severe, are uncomfortable and can lead the patient to discontinue treatment. The most common adverse effect is dry mouth and throat, followed by gastrointestinal complaints, headache, dizziness, and blurred vision.19
Transdermal oxybutynin (Kentera, Gebro Pharma) is sold as patches with a surface area of 39cm2 that contain 36mg of oxybutynin and release the active ingredient at a nominal amount of 3.9mg every 24hours The dose recommended in the summary of product characteristics is 2 patches per week, which are applied in rotation on the thigh, abdomen, and hip.
The results of our study show that the symptoms of PHH improve during treatment with transdermal oxybutynin in an acceptable percentage of patients and that adverse effects are infrequent.
In the assessment of the severity of the symptoms of PHH, subjective scales are considered more appropriate than objective methods, since the effect on quality of life not only depends on the severity of HH in quantitative terms, but also on the degree to which each patient adapts to this severity.20 HDSS has been validated in English (not in Spanish) and is scored on a scale of 1 to 4 depending on the intensity of sweating and its interference with daily activities. However, given that the scale has only 4 points, it could lack sensitivity for detecting slight modifications; therefore, it may not reflect some variations in symptoms.21 Solish et al.11 calculated that a 1-point improvement on the HDSS would correspond to an approximately 50% decrease in sweating and that a 2-point improvement would correspond to an 80% decrease. Therefore, we decided to complement the HDSS with a VAS in which the patient grades the intensity of HH on a scale of 1 to 10 (minimum grade, 1; maximum grade 10) in order to provide a more accurate appreciation of the improvement and thus increase the sensitivity of the evaluation of the response to treatment. Analysis of the results of our study shows that patients whose HDSS score did not change did in fact improve according to the VAS. This finding is consistent with the belief that decreases in sweating of up to 50% will not be recorded by the HDSS.
In our study, the HDSS score improved in 60% of patients during treatment, as follows: 1 point, 60%; 2 points, 33.3%; and 3 points, 6.6%. In their prospective placebo-controlled trial involving 62 patients with PHH treated with oral oxybutynin at 7.5mg/d, Schollhammer et al.21 recorded a 60% improvement in the HDSS score (1 point, 28%; 2 points, 61%; 3 points, 11%). Adverse effects were recorded in a considerable percentage of patients and included dry mouth (43%), blurred vision (13%), gastrointestinal complaints (9%), headache (3%), asthenia (3%), and difficulty urinating (3%). Although the rates of improvement with transdermal oxybutynin were not as high as those reported for the oral formulation in our study, the adverse effect profile was better, with only 8% of patients affected (mild to moderate irritant contact dermatitis).
We must take into account that the doses generally used for treatment of PHH with oral oxybutynin in the literature range from 2.5mg to 10mg per day.19 The dose of transdermal oxybutynin that is released is calculated to be 3.9mg per day, which, while within the appropriate range, can be considered low. Compared with studies based on larger doses of oral oxybutynin, the low dose of the transdermal patch formulation could lead to a poorer response in the case of excessive sweating, although, even so, our data indicate that efficacy is acceptable with the “low dose” released by the patches. Studies on management of overactive bladder in which higher doses are administered (1 daily patch or 2-3 patches applied simultaneously with 2 changes per week) report better control of symptoms and a favorable safety profile.22,23 If these data are extrapolated, we could consider increasing the dose of the drug in cases of PHH that are not controlled with lower doses, although intensifying treatment in this way would likely lead to an increase in the incidence of adverse effects.
The monthly cost of aluminum salts, which are marketed in various formulations, is €12-18. The treatment is not covered by the Spanish National Health System. The monthly cost of transdermal oxybutynin is €40.59 compared with €4.40 for the oral formulation (at a dose of 10mg per day). Both formulations are covered, albeit for different indications.
The main limitations of this study are the number of cases included and the lack of a control group with which to compare the results of treatment.
An exhaustive review of the literature revealed no other reports of cases of PHH treated with transdermal oxybutynin.
Although the experience we report here is limited and broader placebo-controlled studies are necessary to confirm or refute our data, the preliminary findings we present indicate that transdermal oxybutynin has an excellent safety profile for the management of PHH and can be considered a valid option for treatment.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purposes of this study.
Confidentiality of dataThe authors declare that they have followed their insitutional protocols on publication of patient data.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bergón-Sendín M, Pulido-Pérez A, Sáez-Martín LC, Suárez-Fernández R. Experiencia inicial con oxibutinina transdérmica en el tratamiento de la hiperhidrosis. Actas Dermosifiliogr. 2016;107:845–850.