Photodynamic therapy and imiquimod are highly regarded treatments dermatologists frequently prescribe for actinic keratoses, basal cell carcinoma, and Bowen disease. The scarcity of evidence from comparative trials prevents us from drawing well-founded conclusions about the efficacy, tolerance, and adverse effects of these therapeutic options or to recommend one over the other in any particular type of lesion or patient. On the other hand, in certain conditions (eg, actinic chelitis, immunosuppression, and basal cell carcinoma affecting the eyelids), there is evidence to support the use of photodynamic therapy or imiquimod even though they might initially seem contraindicated. We critically review and compare the use of these 2 treatments in order to suggest which is more appropriate in specific cases.
La terapia fotodinámica (TFD) y el imiquimod son dos excelentes tratamientos utilizados frecuentemente en Dermatología para las queratosis actínicas, el carcinoma basocelular (CBC) o la enfermedad de Bowen. No existen suficientes estudios comparativos entre ellos para poder extraer buenas conclusiones sobre su eficacia, su tolerancia o sus efectos secundarios y para poder situar a un tratamiento por encima del otro en un tipo de lesión o paciente en concreto. Por otra parte, existen situaciones o indicaciones particulares como la queilitis actínica, los pacientes inmunodeprimidos o los CBC localizados en párpados donde estos dos tratamientos pueden considerarse inicialmente contraindicados; sin embargo, existe suficiente evidencia para poder utilizarlos.
Vamos a realizar una revisión de la TFD y el imiquimod, bajo un punto de vista crítico y comparativo entre ellos, para poder ayudar a responder a la cuestión de qué tratamiento es más recomendable en un paciente determinado.
Photodynamic therapy (PDT) and imiquimod are 2 highly regarded treatments with very similar indications in dermatology. While many studies have compared one or other of these therapies with other treatment options—for instance, cryotherapy,1–3 fluorouracil,3,4 or surgery5,6—very few have compared PTD with imiquimod.7,8 It is not unusual in routine clinical practice for clinicians to have difficulty deciding which of these therapies is more appropriate in a specific case since both are routinely used to treat actinic keratoses (AKs), basal cell carcinoma (BCC), and Bowen disease (BD). Several small case series and anecdotal case reports have described the use of PDT and/or imiquimod in other skin cancers, including lentigo maligna,9 mycosis fungoides,10 and Paget disease,11 as well as in benign skin diseases.12 However, the lack of scientific evidence for their use in these settings and the existence of more appropriate treatment options make it unlikely that the list of approved indications for PDT or imiquimod will be modified, a step that would allow these treatments to be used more often and with greater safety.
The initial step in PDT is the application of a photosensitizing agent, either aminolevulinic acid (ALA) or methyl-aminolevulinic acid (MAL), to the lesion to be treated. This is then incubated under an occlusive dressing for at least 3hours and subsequently illuminated, usually with a light having a wavelength in the 570 to 670nm range. PDT with MAL (Metvix cream, Galderma SA) is approved for AKs, superficial and nodular BCC, and BD according to the Summary of Product Characteristics (SPC). The therapeutic indication in the case of AKs is nonhyperkeratotic lesions on the face or scalp. The recommended regimen is a single session, which can be repeated after a 3-month interval if needed. In the treatment of BD and superficial and/or nodular BCC, 2 PDT sessions separated by an interval of at least 1 week are recommended.
Imiquimod is sold in the form of a 5% cream (Aldara cream, MEDA AB), which is applied by the patient. It is approved for the treatment of condylomata acuminata, nonhyperkeratotic, nonhypertrophic AKs on the face or scalp in immunocompetent patients, and small superficial BCCs (the SPC does not specify the size). The posology specified in the case of AKs is once-daily application 3 times a week for 4 weeks. If no response is observed on follow-up, the 4-week treatment cycle may be repeated. In the treatment of superficial BCCs, imiquimod should be applied 5 times a week for 6 weeks.
We review the use of PDT and imiquimod in the approved indications. We also discuss their use in other settings in which they might initially appear to be contraindicated, such as actinic cheilitis, tumors in transplant recipients, and BCCs on the eyelids. The safety and efficacy of these treatments in these settings have, however, been demonstrated, making it possible to consider their use in selected cases. The benefits and drawbacks of the 2 treatments are analyzed to provide a basis for decisions regarding the most appropriate choice of treatment in a given situation.
Actinic KeratosesAKs are the precancerous lesions most frequently encountered by dermatologists. They often appear in the context of field cancerization. This term is used to describe areas of chronically exposed skin characterized by the presence of AKs and histologic changes. In addition to dysplastic keratinocytes, the histologic features include molecular changes such as p53 mutations that predispose patients to squamous cell carcinomas but are not manifest in the form of clinically apparent lesions.13,14 PDT and imiquimod are the first-line treatments in these patients as they can be used to treat large areas of skin and subclinical lesions at the same time. The cosmetic results obtained with both treatments are also excellent.3,15,16 However, there are some differences that may influence the decision to use one treatment or the other.
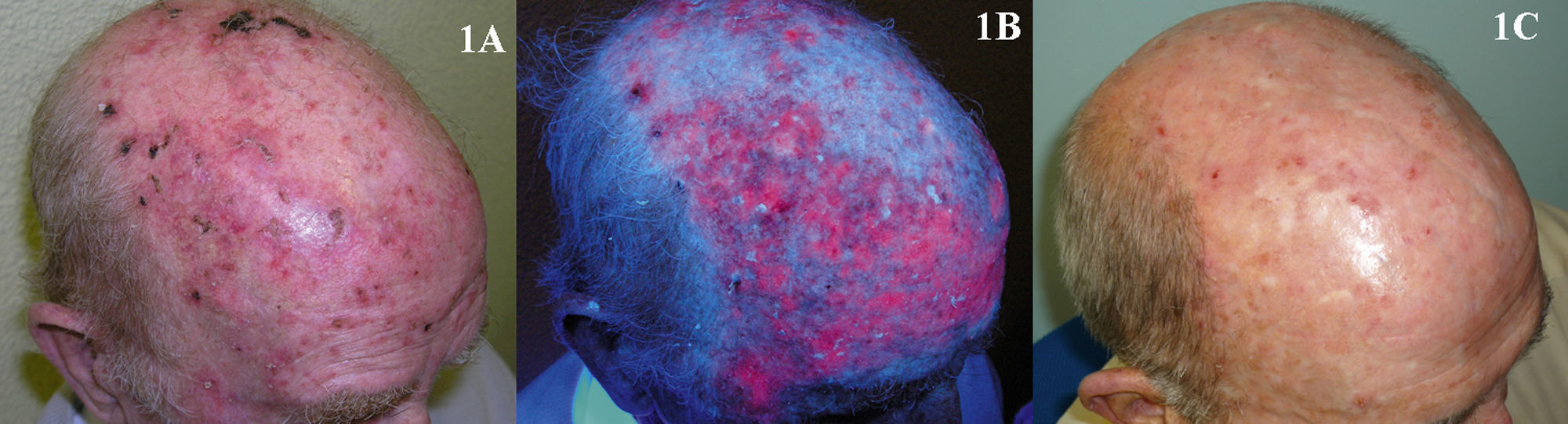
The use of PDT in the treatment of AKs has been studied by numerous authors, but it is difficult to accurately assess overall treatment efficacy because of differences in the methodologies used in each study. Cure rates after 1 or 2 treatment sessions using MAL as the photosensitizing agent were 69% to 91% (Fig. 1).15,17–20 The adverse effect most often reported with PDT is pain during the illumination phase of treatment. Pain tends to be more severe in patients with skin phototypes I and II and when the lesions treated are on the head.21 However, as in any other dermatologic procedure, there are a variety of effective methods for controlling or reducing pain, including cold air and nerve block anesthesia.22 Thus, the pain caused by PDT should not be a reason for avoiding the use of this treatment modality. Interestingly, despite the widespread impression that its tolerance can be problematic, PDT is the highest-rated treatment for AKs in terms of patient satisfaction in all the studies that have evaluated this parameter.1,8,15,19,23 This high degree of patient satisfaction is probably due to the fact that a PDT session takes up very little time and evidence of improvement can be observed within a few weeks. Likewise, PDT is not a treatment patients have to apply themselves and is therefore a modality usually not associated with doubts on the part of the patient or the occurrence of adverse effects at home which they are unable to cope with.
It is difficult to evaluate the long-term recurrence rate in patients with AKs treated with PDT because very few of the studies in the literature have a follow-up period longer than 12 months.24–26 Rates of up to 24% at 1 year have been reported,27 but recurrence is lower with fractionated illumination28,29 and nonhyperkeratotic lesions.30
In transplant recipients, PDT has proved very useful in curing AKs31,32 and in preventing new lesions.33,34 Moreover, some studies have shown cyclic application of PDT to reduce the risk of squamous cell carcinoma in these patients.35 However, other authors have demonstrated that PDT does not reduce the incidence of squamous cell carcinomas in transplant recipients with actinic damage.36
The safety and efficacy of PDT in the treatment of actinic cheilitis (Fig. 2) have been demonstrated in many studies. Good response and good tolerance have been reported following 2 or 3 PDT sessions with either MAL37,38 or ALA,39,40 leading some authors to consider PDT the treatment of choice in actinic chelitis.41 However, in the studies of PDT in which histologic confirmation of response was obtained, lesions persisted in 20% to 53% of patients37,42 and histologic changes compatible with actinic cheilitis were found after 18 months of follow up in 34.6% of the patients treated.40 Based on the evidence cited above, PDT as a treatment for actinic cheilitis has a B strength recommendation rating and should be considered a second-line treatment.43
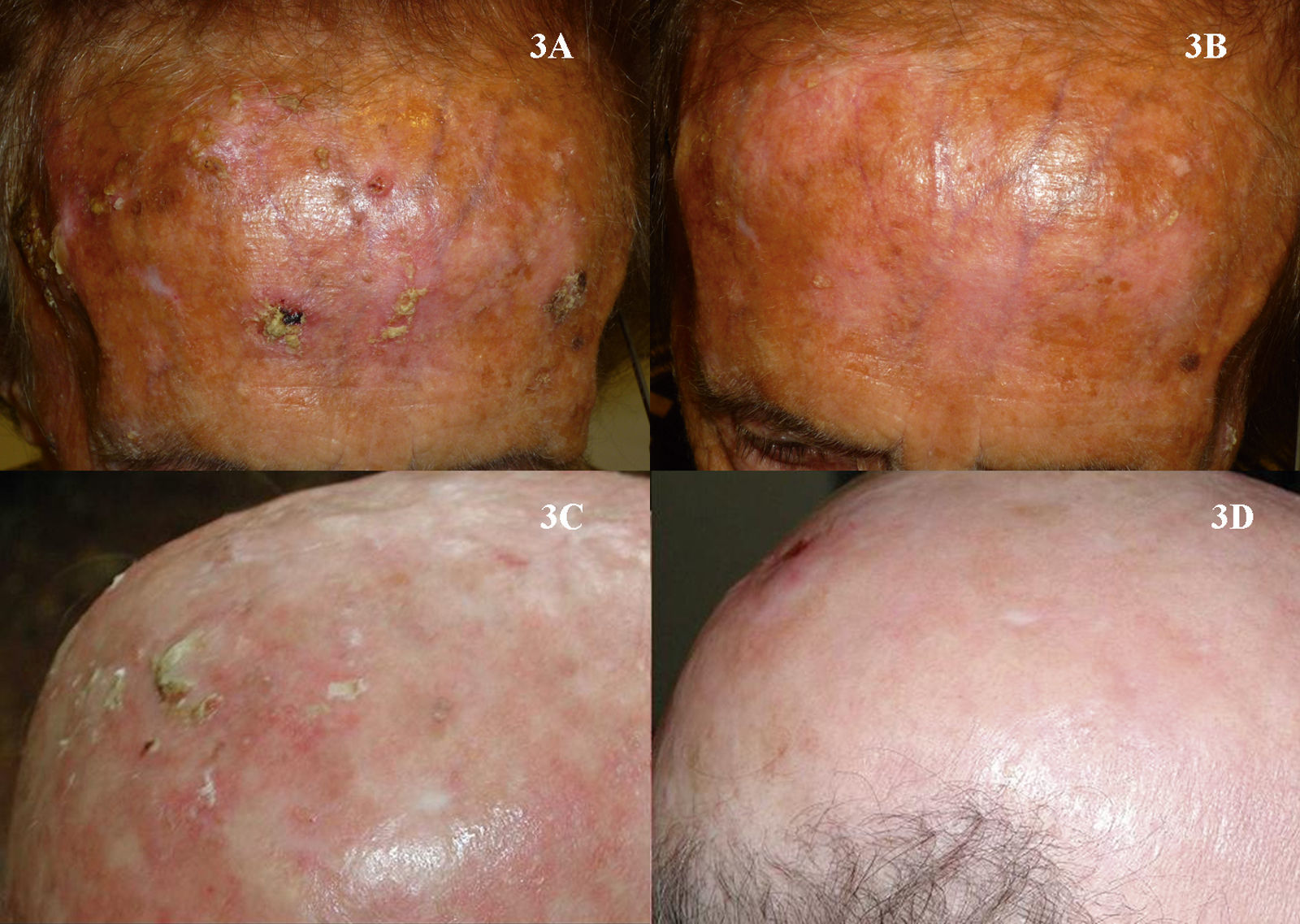
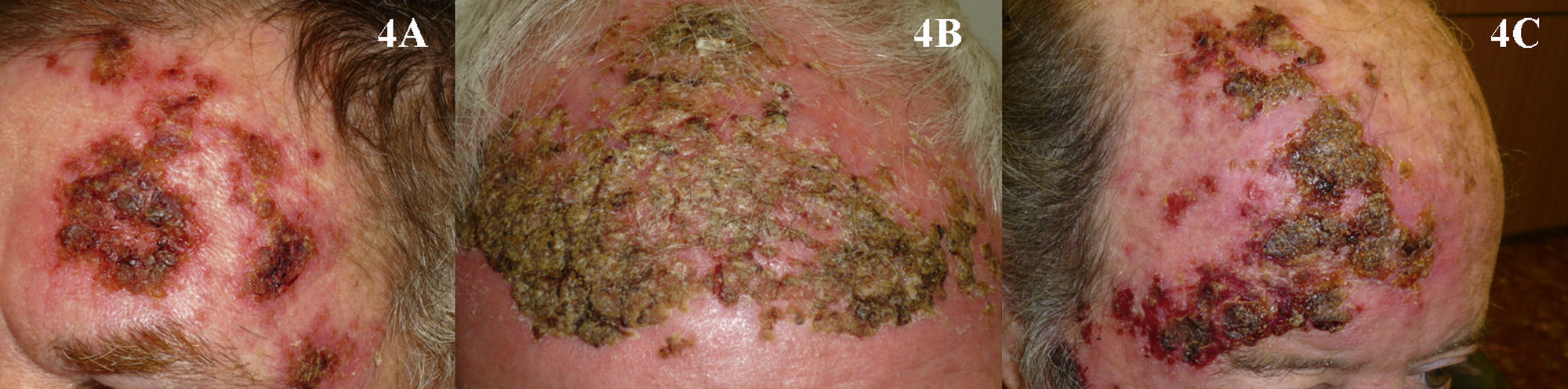
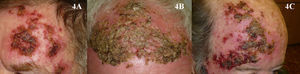
It is likewise difficult to determine the exact cure rate achieved with imiquimod in the treatment of AKs. Most studies report complete or partial clinical response rates, while others report histologic response. Furthermore, treatment regimens vary greatly from one study to another. Three meta-analyses have found imiquimod to be a very effective treatment for AKs, achieving complete response (100% of the lesions resolved after treatment) in more than 70% of patients (Fig. 3).44–46 One of the most important characteristics of imiquimod treatment is the high negative predictive value of the assessment of the clinical response. The methodology used in many studies of imiquimod appears to be more rigorous than that used in studies of PDT, and histologic confirmation of treatment response is more often included. The probability that AKs that are clinically resolved with imiquimod are also histologically resolved ranges from 86% to 100%.3,47–49 It is interesting to note that studies of imiquimod in AK have longer follow-up periods than studies of PDT in this setting. More imiquimod studies have assessed clinical response 1 year after completion of treatment, when most authors report a recurrence rate close to 10%,3,48,50 although Jorizzo et al.51 reported a recurrence rate of 39% at 1 year. The main adverse effect associated with imiquimod is a local inflammatory reaction that occurs during treatment. This reaction, which is necessary and even predictive of a good reponse,44 appears within a few days of treatment initiation and takes the form of erythema, inflammation, swelling, and even crusting (Fig. 4). In addition, some patients develop flu-like symptoms, such as general malaise, fever, and muscle weakness. These adverse effects appear to be the main reason why some clinicians are reluctant to prescribe imiquimod to patients with AKs. The treatment cycle lasts for 4 weeks, and the local reaction may last as long as 2 months. Thus, patients and their families need to be properly informed so that they will understand the process involved. This precaution will also prevent unnecessary phone calls, unscheduled visits to the physician, and even visits to the emergency department, where symptoms are often misinterpreted and treatment discontinued. This adverse reaction, which has been widely studied in protocols and studies, has been reported in almost 100% of patients in the majority of publications.44,46–48 Paradoxically, however, most authors have concluded that imiquimod is well tolerated in the treatment of AKs.48,52,53 In the only 2 studies comparing PDT and imiquimod that have evaluated patient preference7 and satisfaction,8 more patients favored PDT. Researchers investigating ways to reduce the local adverse effects of imiquimod while maintaining its efficacy have evaluated creams with reduced concentrations of the active ingredient—2.5% or 3.75%—with promising results.54,55 However, these formulations are not currently available in Europe.
According to the SPC, the use of imiquimod is contraindicated in transplant recipients because, in theory, the drug's mechanism of action relies on the patient having an adequate immune system. Nevertheless, some studies of the safety and efficacy of imiquimod in transplant recipients have reported more than acceptable results in the treatment of AKs,56,57 and even in preventing the development of squamous cell carcinoma.58 Moreover, there is scant evidence in the literature to suggest that imiquimod may produce local or systemic immunologic changes in the patient, skin tumors in the treatment area,59 or autoimmune disorders.60 Most studies and reviews of the literature accept and recommend the use of imiquimod in immunosuppressed patients.57,61
Imiquimod may also be considered in the treatment of actinic cheilitis, although there are fewer studies on its use in this setting than in the case of PDT. According to the 2 largest series of patients studied (1562 and 563), imiquimod appears to be clinically and histologically effective when applied for at least 4 weeks. However, the use of imiquimod on the lips is greatly limited by the visible local reaction that almost always accompanies treatment and the possibility that the patient may develop oral aphthous ulcers64 or experience an outbreak of herpes simplex43 during treatment.
In conclusion, both PDT and imiquimod are the best treatments available for patients with multiple AKs or field cancerization. Their efficacy is similar although there are certain differences that should be taken into account when deciding which option is most suitable in a particular patient or situation. PDT is superior in terms of tolerance and patient satisfaction, but imiquimod appears to be associated with lower recurrence. While both PDT and imiquimod are effective in the treatment of AKs in immunosuppressed transplant recipients, PDT would appear to be a slightly better choice because of its mechanism of action and the larger number of studies supporting its efficacy in curing AKs and in preventing both new lesions and the development of squamous cell carcinomas. In the case of actinic cheilitis, imiquimod is not recommended because of the associated adverse effects; PDT, by contrast is a recommendable alternative in many patients but surgical treatment remains the method of choice in this setting.
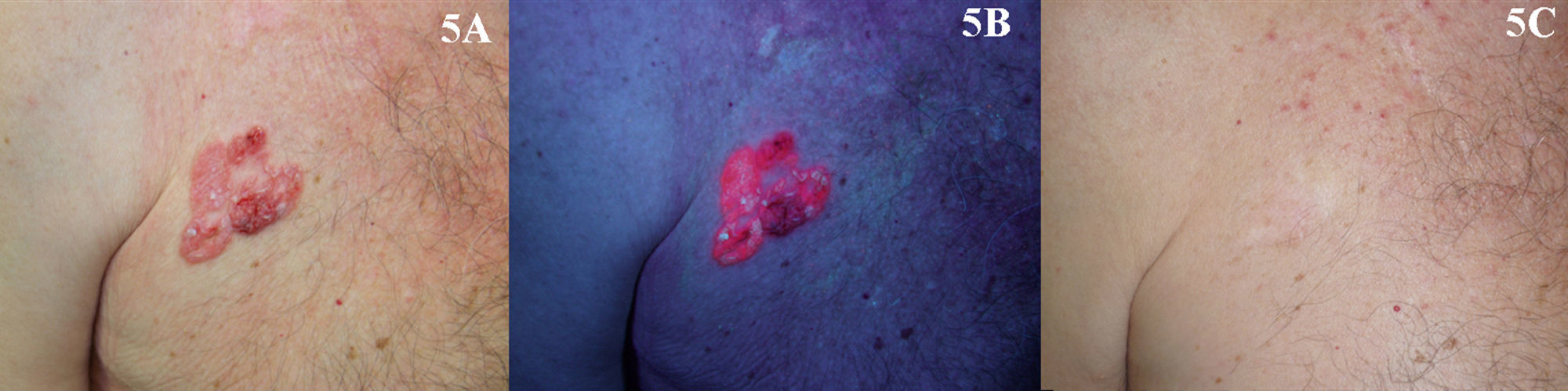
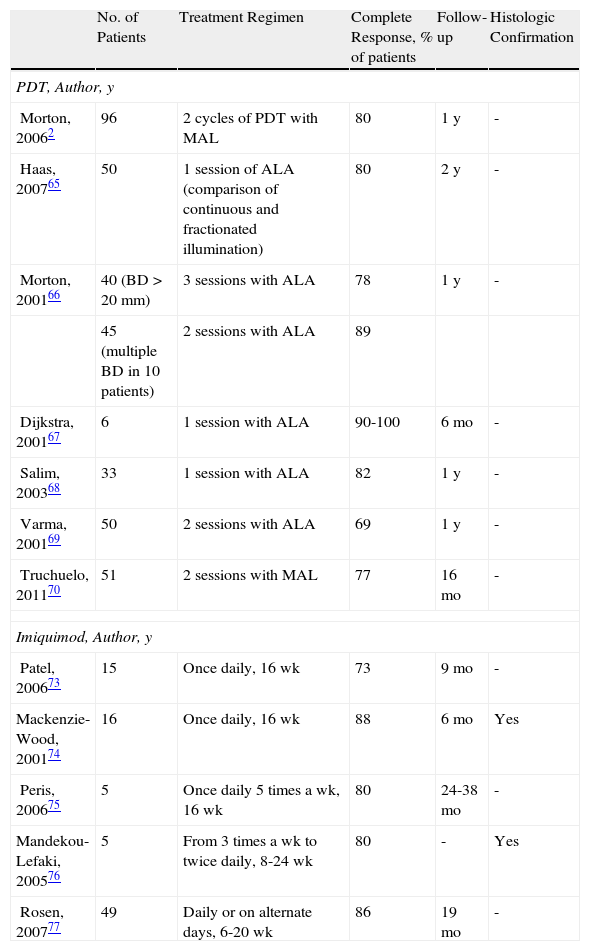
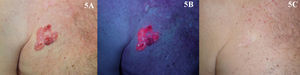
Bowen DiseaseThe efficacy of PDT in the treatment of BD is between 69% and 100% (Fig. 5).2,65–70 Most of the studies reviewed involved 2 treatment sessions and used ALA as the photosensitizer. In the largest multicenter study in the literature, Morton et al.2 reported an 80% complete response rate at 12 months following 2 sessions of PDT with MAL. Many studies report excellent cosmetic results in most cases.2,70 Given that these lesions are usually small (only a few centimeters) and the fact that they are not located on the head, tolerance of treatment is generally very good.21,71 The British Association of Dermatologists (BAD) guidelines for the management of BD has assigned PDT the highest rating of all the treatment options reviewed (quality of evidence rating I and strength of recommendation A).72
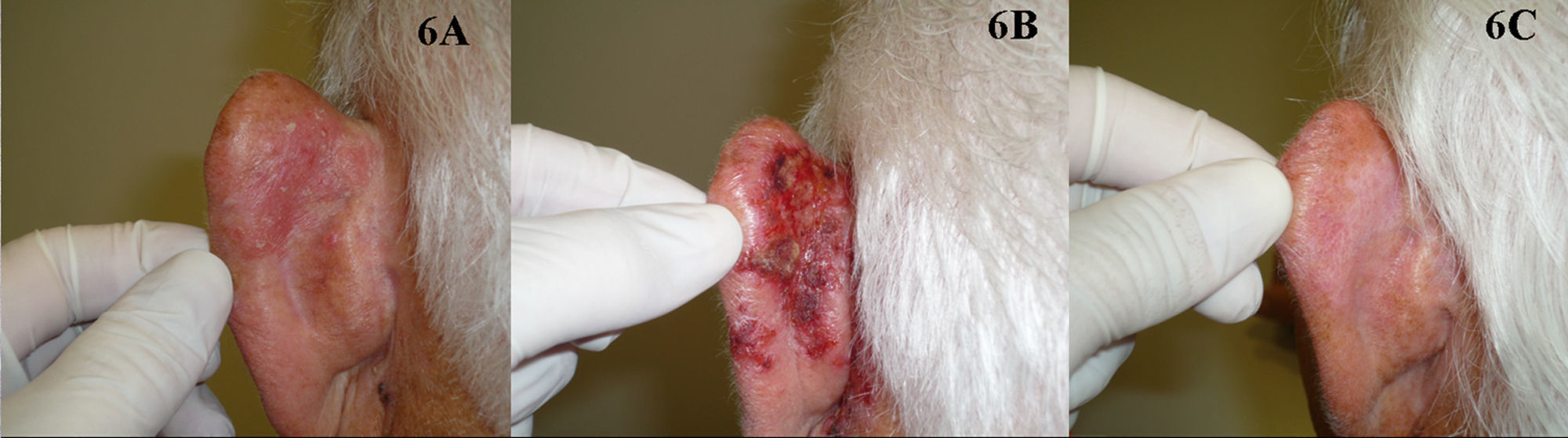
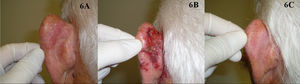
Five studies of patients with BD treated with imiquimod have been published (Fig. 6).73–77 Overall, treatment lasted from 9 to 16 weeks and complete response was obtained in 73% to 88% of the patients studied, in some cases with histologic confirmation; the longest follow-up period was 19 months. Tolerance was generally acceptable, although a few patients discontinued treatment because of the local reaction. The BAD guidelines assigned treatment with imiquimod a quality of evidence rating of I and a strength of recommendation rating of B.72
Table 1 lists the most important studies of PDT and imiquimod in the treatment of BD.
Studies of Patients With Bowen Disease Treated With Photodynamic Therapy or Imiquimod.
| No. of Patients | Treatment Regimen | Complete Response, % of patients | Follow-up | Histologic Confirmation | |
| PDT, Author, y | |||||
| Morton, 20062 | 96 | 2 cycles of PDT with MAL | 80 | 1 y | - |
| Haas, 200765 | 50 | 1 session of ALA (comparison of continuous and fractionated illumination) | 80 | 2 y | - |
| Morton, 200166 | 40 (BD>20mm) | 3 sessions with ALA | 78 | 1 y | - |
| 45 (multiple BD in 10 patients) | 2 sessions with ALA | 89 | |||
| Dijkstra, 200167 | 6 | 1 session with ALA | 90-100 | 6 mo | - |
| Salim, 200368 | 33 | 1 session with ALA | 82 | 1 y | - |
| Varma, 200169 | 50 | 2 sessions with ALA | 69 | 1 y | - |
| Truchuelo, 201170 | 51 | 2 sessions with MAL | 77 | 16 mo | - |
| Imiquimod, Author, y | |||||
| Patel, 200673 | 15 | Once daily, 16 wk | 73 | 9 mo | - |
| Mackenzie-Wood, 200174 | 16 | Once daily, 16 wk | 88 | 6 mo | Yes |
| Peris, 200675 | 5 | Once daily 5 times a wk, 16 wk | 80 | 24-38 mo | - |
| Mandekou-Lefaki, 200576 | 5 | From 3 times a wk to twice daily, 8-24 wk | 80 | - | Yes |
| Rosen, 200777 | 49 | Daily or on alternate days, 6-20 wk | 86 | 19 mo | - |
aComplete response rates refer to the result at the end of the follow-up period specified.
Abbreviations: ALA, aminolevulinic acid; BD, Bowen disease; MAL methyl aminolevulinate.
In the treatment of BD, PDT is generally preferred over imiquimod for a number of reasons, as indicated in the BAD guidelines for this disease.72 PDT is superior to imiquimod in this setting, first because, strictly speaking, it is approved for this indication while imiquimod is not. Furthermore, more patients have been studied in the case of PDT and the rate of complete response to treatment is slightly higher. Nonetheless, treatment with both PDT and imiquimod should be considered before surgical intervention in patients with BD. It would seem unreasonable to propose surgery when 2 conservative choice of treatment should take into account the site of the lesion (for example, in the case of BD affecting the perianal region, the ears, or other areas where illumination may be complicated), the availability of the patient to attend PDT sessions or, in the case of imiquimod, the patient's ability to properly apply treatment.
Basal Cell CarcinomaA review of studies of PDT in the treatment of BCC also reveals methodological differences between studies. Some authors have used ALA and others MAL. The light sources and the methods used to apply the light also differ, as do methods for assessing response. However, there is consensus on the recommended regimen of 2 sessions of PDT with ALA or MAL separated by an interval of at least 1 week. It is also generally accepted that superficial BCC responds very well to 2 sessions of PDT using ALA or MAL. Response rates of 81% to 97% were reported for these treatments in the largest case series.67,78–87 On the basis of this evidence, the BAD guidelines recommend PDT as the first-line choice for the treatment of BCC (quality of evidence I and strength of recommendation A).88 However, the response rates reported for nodular BCC are lower than for superficial BCC, with complete response in between 20% to 94% of patients depending on the study.5,67,82–86,89 Furthermore, it could be said that these figures do not entirely reflect the real efficacy of PDT because—in the studies reporting the best results—lesions were pretreated with various procedures, such as the application of dimethyl sulfoxide,86 debulking,5 curettage,86 or shaving of the tumor.85 Furthermore, a study of recurrence during follow-up periods ranging from 1 to 6 years reveals a recurrence rate for superficial BCC of between 3% and 22%78,80,84–86 and a somewhat higher rate (14%-33%) for nodular BCC.5,84–86,89 Consequently, PDT is considered to be a second-line treatment for nodular BCC, (quality of evidence I, strength of recommendation B) with surgery as the first-line treatment (quality of evidence I, strength of recommendation A).88
Another interesting conclusion that can be drawn from the findings of the studies on PDT in BCC is that fractionation of the illumination phase into 2 exposures separated by a dark interval of varying length greatly improves response; this conclusion has relatively broad acceptance.5,29,78,87
Tables 2 and 3 list the most important studies of PDT and imiquimod in the treatment of superficial and nodular BCC.
Studies of Superficial Basal Cell Carcinoma Treated With Photodynamic Therapy or Imiquimod.
| No. of Patients | Treatment Regimen | Complete Response, % of patients | Follow-up | Histologic Confirmation | |
| PDT, Author, y | |||||
| Dijkstra, 200167 | 33 | 1 session with ALA | 82 | 6 mo | - |
| Haas, 200678 | 505 | 1 session with ALA (comparing continuous and fractionated illumination) | 89-97 | 1 y | - |
| Soler, 200079 | 245 | 1 session with prior application of DMSO using ALA and 2 types of light | 82-86 | 6 mo | - |
| Szeimies, 200880 | 100 | 2 sessions with MAL | 90 | 1 y | - |
| Basset-Seguin, 200881 | 114 | 1 session with MAL | 81 | 3 mo | - |
| Fantini, 201182 | 116 | 2 sessions with MAL | 82 | - | - |
| Weenberg, 199683 | 157 | 1 session with ALA | 92 | 6 mo | Yes |
| Vinciullo, 200584 | 80 | 2 sessions with MAL | 82 | 2 y | Yes |
| Horn, 200385 | 49 | 2 sessions with MAL | 92 | 3 mo | - |
| Christensen, 200986 | 60 | 1 or 2 sessions with ALA and prior application of DMSO | 81 | 6 y | Yes |
| Star, 200687 | 67 | 1 session with ALA using 2 types of light | 84 | 5 y | - |
| Imiquimod, Author, y | |||||
| Geisse, 200295 | 31 | Once daily, 12 wk | 87 | 6 mo | Yes |
| Marks, 200196 | 33 | Once daily, 6 wk | 88 | 6 wk | Yes |
| Schulze, 200597 | 84 | Once daily, 6 wk | 80 | 12 wk | Yes |
| Geisse, 200498 | 179 | Once daily, 6 wk | 79 | 12 wk | Yes |
| Marks, 200499 | 97 | Once daily, 6 wk | 77 | 12 wk | Yes |
| Quirk, 2006100 | 169 | Once daily, 6 wk | 82 | 2 y | - |
| Gollnick, 2005101 and 2008102 | 182 | 5 times a wk, 6 wk | 69 | 5 y | - |
| Peris, 2005103 | 30 | 3 times a wk, 6 wk | 91 | 23 mo | - |
| Quirk, 2010104 | 169 | Once daily, 6 wk | 80 | 5 y | - |
| Ruiz-Villaverde, 2009105 | 82 | 3 times a wk, 4 wk | 85 | 2 y | - |
| Daudén, 2011106 | 446 | 5 times a wk, 6 wk | 83 | - | - |
| Shumack, 2004107 | 66 | 5 times a wk, 6 wk | 83 | 12 wk | Yes |
aComplete response rates refer to the result at the end of the follow-up period specified.
Abbreviations: ALA, aminolevulinic acid; DMSO, dimethyl sulfoxide; MAL, methyl aminolevulinate.
Studies of Patients with Nodular Basal Cell Carcinoma Treated With Photodynamic Therapy and Imiquimod.
| No. of Patients | Treatment Regimen | Complete Response, % of Patients | Follow-up | Histologic Confirmation | |
| PDT, Author, y | |||||
| Mosterd, 20055 | 85 | 1 session with ALA after partial debulking of the tumor | 94 | 3 mo | - |
| Dijkstra, 200167 | 8 | 1 session with ALA | 50 | 3-12 mo | - |
| Fantini, 201182 | 78 | 2 sessions with MAL | 33 | - | - |
| Wennberg, 199683 | 10 | 1 session with ALA | 20 | 6 mo | Yes |
| Vinciullo, 200584 | 33 | 2 sessions with MAL | 67 | 2 y | Yes |
| Horn, 200385 | 52 | 2 sessions with MAL after shave excision of the nodular component | 75 | 3 mo | Yes |
| Christensen, 200986 | 36 | 1 or 2 sessions with ALA and DMSO following curettage | 81 | 6 y | Yes |
| Rhodes, 200789 | 53 | 2-3 sessions with MAL | 92 | 3 mo | - |
| Imiquimod, Author, y | |||||
| Peris, 2005103 | 19 | 3 times a wk, 12 wk | 53 | 2 y | - |
| Shumack, 2002108 | 137 | From twice daily 7 d a wk to 3 times a wk, 6 to 12 wk | 42-76 | 6 wk | Yes |
| Schiessl, 2007109 | 26 | 5 times a wk, 6 wk | 88 | 6 wk | Yes |
| Huber, 2004110 | 15 | 3 times a wk, 12 wk | 100 | 15 wk | Yes |
| Eigentler, 2007111 | 101 | 3 times a wk, 10 wk | 57 | 8 wk | Yes |
| Sterry, 2002112 | 90 | 2 or 3 times a wk, 6 wk | 50-65 | 6 wk | Yes |
| Wu, 2006113 | 34 | Daily for 6-10 wk with prior curettage | 94 | - | Yes |
aComplete response rates refer to the result at the end of the follow-up period specified.
Abbreviations: ALA, aminolevulinic acid; DMSO, dimethyl sulfoxide; MAL, methyl aminolevulinate.
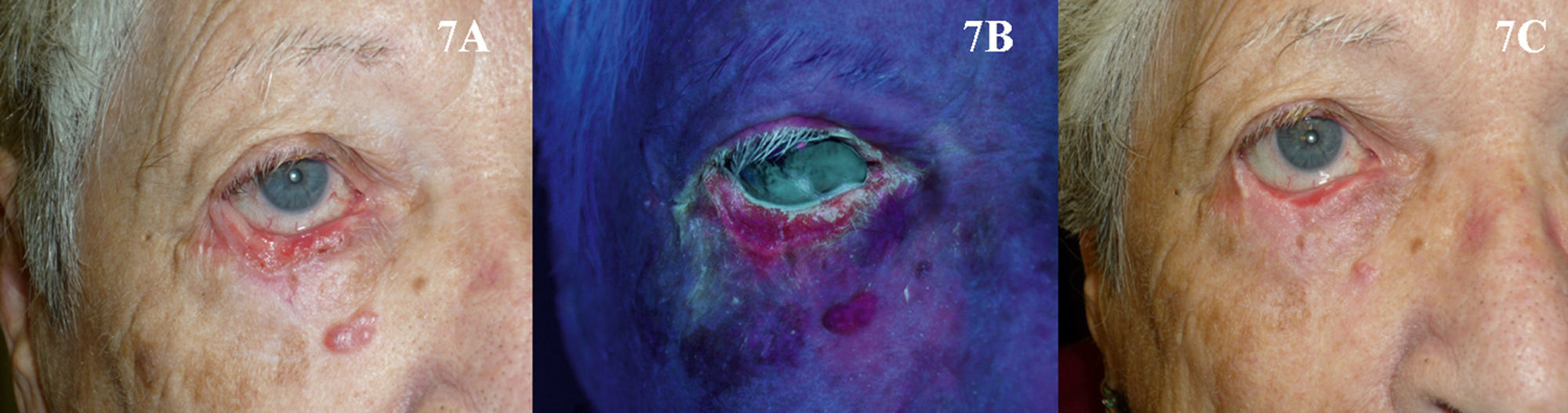
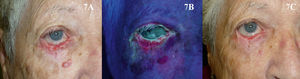
The use of PDT in the periocular area, and even to treat tumors located on the eyelids, might initially appear to be absolutely contraindicated because of the possibility of patient discomfort and the technical difficulties involved. However, based on the available evidence90–93 and our own experience, our opinion is that PDT can be used in selected patients with superficial and nodular BCC in these areas when surgery is contraindicated, but only if the eye is shielded with a protective lens before the photosensitizer is applied (Fig. 7). Tolerance in such cases is generally good, and PDT can be used as a neoadjuvant, palliative, or even curative therapy.
The treatment of superficial BCC with imiquimod has been widely studied. A review of the literature indicates that the methodology used in these studies appears to have been more rigorous and uniform than that used in studies of PDT. In superficial BCC, there are also slightly more studies on the use of imiquimod than of PDT.94 In the largest studies, the rate of complete response in cases of superficial BCC treated with imiquimod ranged from 69% to 91%95–107 (quality of evidence I and strength of recommendation A).88 In most of these studies, response to treatment was confirmed by histologic evidence95–99,107 or analyzed in a follow-up period of up to 5 years,101,102,104 enhancing the reliability of the results regarding the efficacy of imiquimod in the treatment of superficial BCC. However, as occurs in the case of AKs, treatment of BCC with imiquimod causes a local reaction that may last for up to 2 months; this adverse effect often prevents the patient from correctly completing treatment and thus limits the use of this therapy in BCC.
Although imiquimod is not approved for the treatment of nodular BCC, there is nonetheless sufficient evidence and clinical experience to support its use. In the largest studies reviewed, treatment with imiquimod resulted in complete response in between 42% and 100% of cases of nodular BCC.103,108–113 As in the case of PDT, prior curettage has been proposed as a way of increasing the efficacy of imiquimod in this setting.113,114
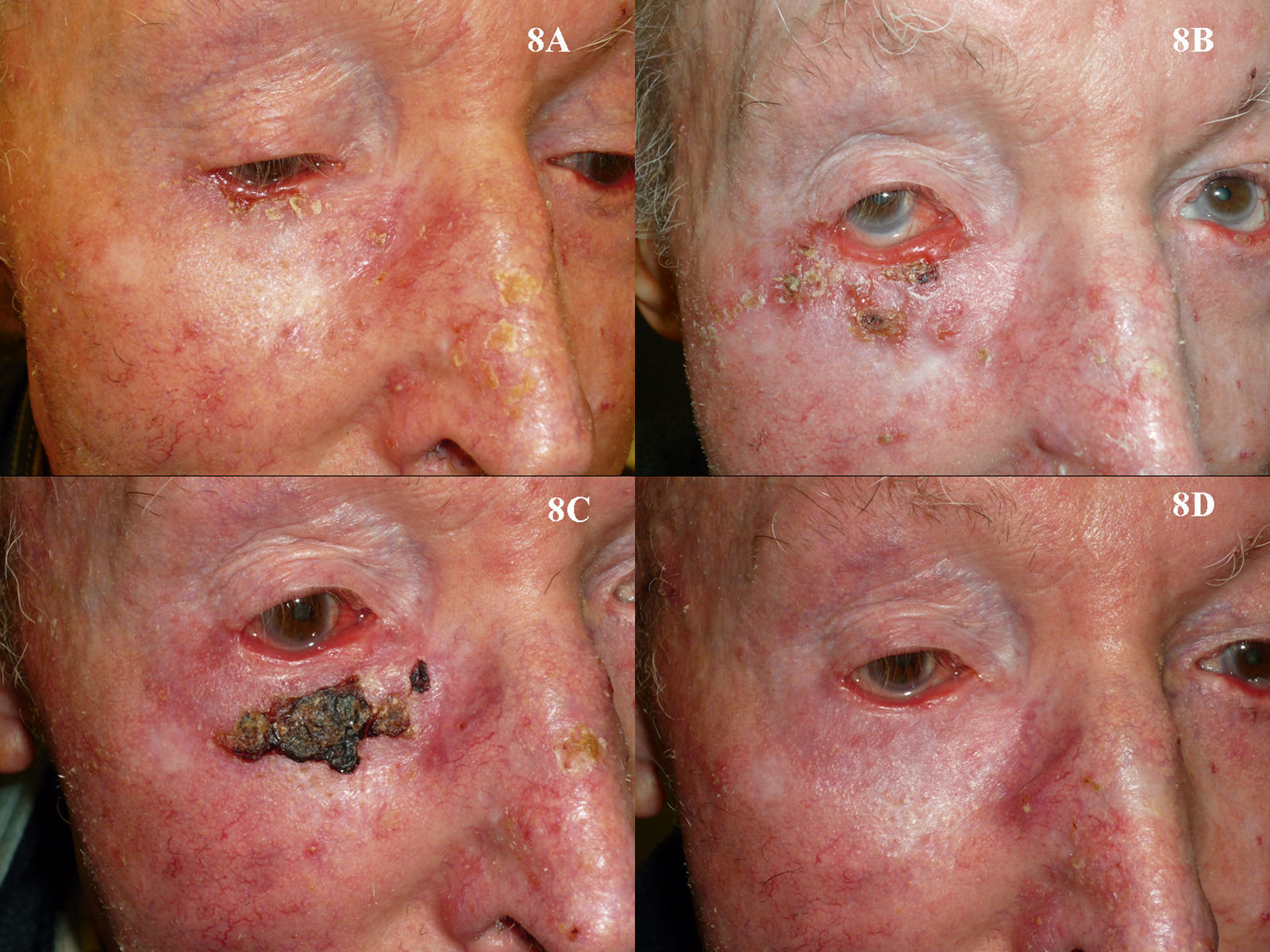
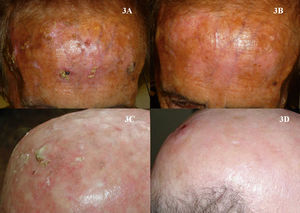
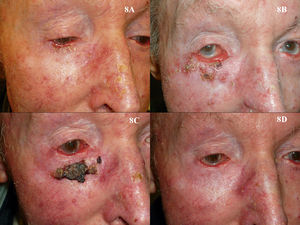
As is the case with PDT, the use of imiquimod to treat BCCs located in a periocular site or on the eyelids might at first appear to be absolutely contraindicated. However, many authors have reported results that support the use of imiquimod in selected cases (Fig. 8).115–119 The studies of García-Martín et al.118 and Cannon et al.119 are of particular interest. García-Martín et al. reported complete clinical and histologic response in all 15 cases of periocular BCC treated with imiquimod, with excellent cosmetic results. Cannon et al. analyzed the adverse effects in 47 patients treated with imiquimod for periocular tumors. The most common effect was conjunctivitis of medium severity (11 patients). All adverse effects resolved once treatment was stopped, and there were no residual adverse effects.
Superficial basal cell carcinoma on the right lower eyelid treated with imiquimod. A, Prior to treatment. B, Local reaction and mild conjunctivitis after 3 weeks of treatment. C, Local reaction and mild conjunctivitis after 6 weeks of treatment. D, Clinical response and resolution of conjunctivitis 1 month after completion of treatment with imiquimod.
Both PDT and imiquimod are less costly options than surgical excision in the treatment of BCC,6,120,121 and PDT is more economical than imiquimod.6
In conclusion, both PDT and imiquimod should be considered first-line treatments for superficial BCC in terms of efficacy. Surgery should only be used when the disease proves refractory to these treatments or when the site of the tumor is a contraindication for these treatments, a specimen is required for histologic examination, or the patient prefers surgery. Several factors come into play in the decision whether to choose PDT or imiquimod to treat superficial BCCs. Taking into account the greater ease of treatment, patient preferences, and the cosmetic results obtained, PDT provides greater benefits and should, therefore, generally be preferred to treatment with imiquimod in this setting.25,88,122 It should be noted that neither PDT nor imiquimod offers greater efficacy than surgery in the treatment of nodular BCC. When surgery is contraindicated in nodular BCC, PDT is rated as a better choice of treatment than imiquimod in the BAD guidelines for the management of BCC and in the SPCs of both products. Finally, in selected patients, both PDT and imiquimod can be attractive alternatives for the treatment of nodular and superficial BCCs on the eyelids and in the periocular area.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: C. Serra-Guillén, E. Nagore, C. Guillén. Terapia fotodinámica versus imiquimod. Actas Dermosifiliogr. 2012;103:488-501.