We report the case of a 74-year-old woman with a history of hypertension, diabetes mellitus, dyslipidemia, spondyloarthritis, and idiopathic thrombocytopenic purpura for which she had undergone splenectomy 5 years previously. She was being followed up by her rheumatologist for joint pain of inflammatory origin with low titers of rheumatoid factor and was taking lorazepam, ranitidine, domperidone, indapamide, AM3, and ursodeoxycholic acid. She attended our clinic with a pruritic disorder that had begun a week earlier and took the form of well-defined discretely desquamative erythematous papules on her back and, to a lesser extent, on her chest, thighs, and proximal upper arms. She had no blisters or mucosal lesions. These signs first appeared during winter, and the patient reported no previous sun exposure. Of interest, she reported starting esomeprazole and a vitamin complex (B1, B6, and B12) 3 weeks before the lesions first appeared. A biopsy specimen of one of the lesions was obtained, and the patient was prescribed mometasone cream twice daily. Esomeprazole and the vitamin supplement were withdrawn.
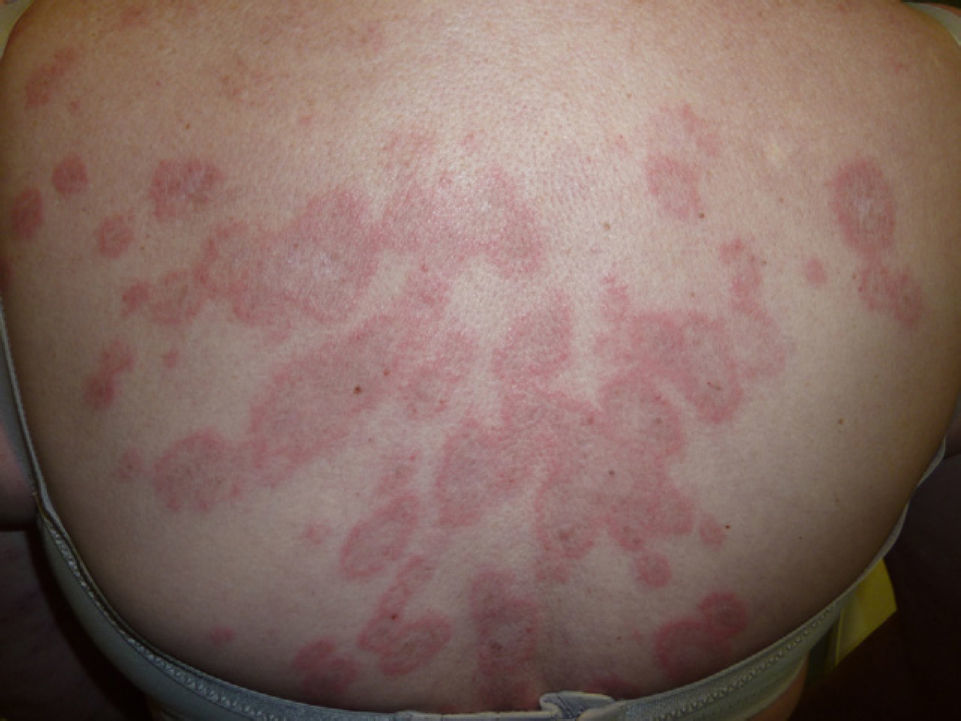
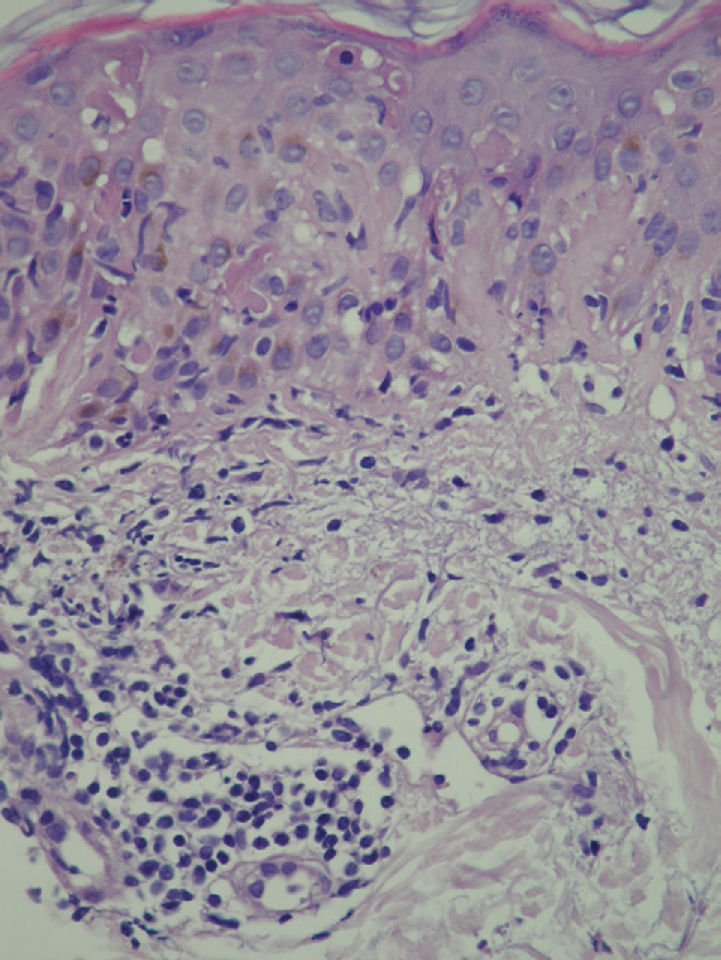
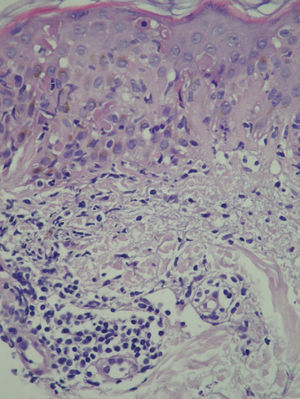
At 3 weeks, the lesions had progressed to confluent annular erythematous-violaceous plaques on the above-mentioned sites (fig. 1). The biopsy revealed hyperkeratosis in the epidermis, epidermal atrophy, degeneration of the basal layer, lymphocytic exocytosis, spongiosis, and occasional necrotic keratinocytes. A superficial perivascular lymphohistiocytic inflammatory infiltrate was observed in the dermis (fig. 2). Based on clinical and microscopy findings, the patient was diagnosed with subacute cutaneous lupus erythematosus (SCLE).
The results of a complete blood count and routine biochemistry were unremarkable. The immunology workup revealed positive titers for antinuclear antibody (1/160), anti-SSA/Ro antibody, and anti-SSB/La antibody. These results had also been positive in laboratory studies performed 2 years previously in the rheumatology department.
Based on a suspected diagnosis of SCLE induced or exacerbated by esomeprazole, treatment with a topical corticosteroid was continued and the culprit medication was withdrawn. The lesions had completely resolved 8 weeks after the interruption of esomeprazole.
SCLE is a well-defined subtype of lupus erythematosus characterized by annular or psoriasiform lesions, limited systemic involvement, and the presence of circulating anti-SSA/Ro antibody.1–3 An association has been described between SCLE and various drugs, including thiazides, statins, calcium channel antagonists, phenytoin, griseofulvin, tumor necrosis factor antagonists, terbinafine,1–5 and, recently, proton pump inhibitors such as omeprazole, lansoprazole, and pantoprazole.6–8 Esomeprazole is the S enantiomer of omeprazole and is used to treat gastroesophageal reflux disease. It came onto the market in Spain in 2002.9
Unlike drug-induced systemic lupus erythematosus, which usually involves positive antihistone antibody titers, drug-induced SCLE is generally associated with positive anti-SSA/Ro antibody titers, but not with positive antihistone antibody titers. In some cases, these titers become negative once the culprit agent is withdrawn.7
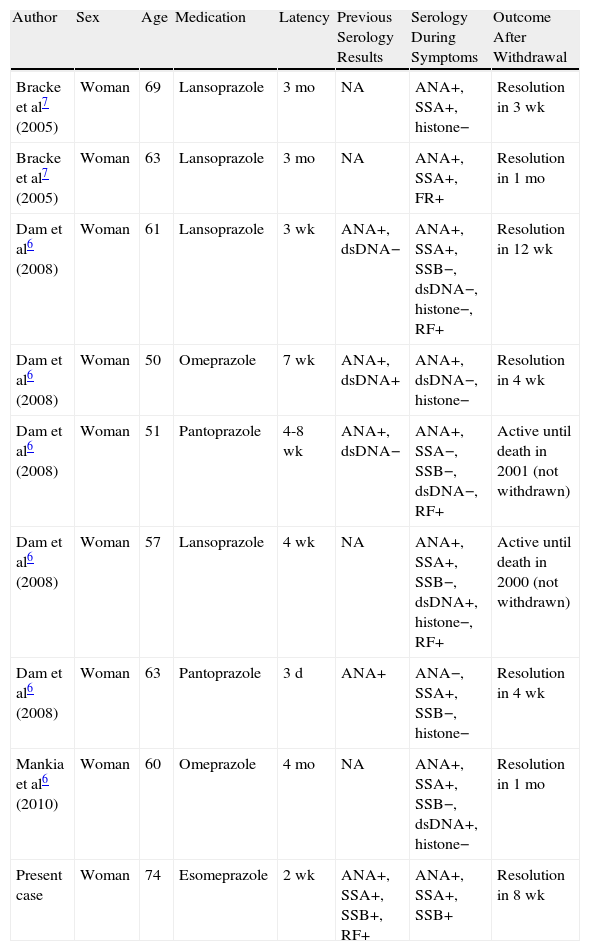
In the case we present, it is striking that anti-SSA/Ro and anti-SSB/La antibody titers were previously positive and that this finding was maintained after onset of the skin symptoms. Four of the 8 previously reported cases of SCLE induced by proton pump inhibitors presented positive antinuclear antibody titers before the onset of cutaneous symptoms; however, it is unknown whether anti-SSA/Ro or anti-SSB/La antibody titers were positive. No analytical results were available for the remaining 4 patients (Table 1).6–8 These findings point to a predisposition to SCLE, which was triggered by proton pump inhibitors. The pathogenic mechanism responsible for the formation of antibodies is unknown. It has been postulated that binding of the drug to proteins could trigger the immune response through a hapten-type mechanism, leading to formation of antibodies.3
Previous Cases and Characteristics of Patients With Subacute Cutaneous Lupus Erythematosus Induced by Proton Pump Inhibitors.
| Author | Sex | Age | Medication | Latency | Previous Serology Results | Serology During Symptoms | Outcome After Withdrawal |
| Bracke et al7 (2005) | Woman | 69 | Lansoprazole | 3 mo | NA | ANA+, SSA+, histone− | Resolution in 3 wk |
| Bracke et al7 (2005) | Woman | 63 | Lansoprazole | 3 mo | NA | ANA+, SSA+, FR+ | Resolution in 1 mo |
| Dam et al6 (2008) | Woman | 61 | Lansoprazole | 3 wk | ANA+, dsDNA− | ANA+, SSA+, SSB−, dsDNA−, histone−, RF+ | Resolution in 12 wk |
| Dam et al6 (2008) | Woman | 50 | Omeprazole | 7 wk | ANA+, dsDNA+ | ANA+, dsDNA−, histone− | Resolution in 4 wk |
| Dam et al6 (2008) | Woman | 51 | Pantoprazole | 4-8 wk | ANA+, dsDNA− | ANA+, SSA−, SSB−, dsDNA−, RF+ | Active until death in 2001 (not withdrawn) |
| Dam et al6 (2008) | Woman | 57 | Lansoprazole | 4 wk | NA | ANA+, SSA+, SSB−, dsDNA+, histone−, RF+ | Active until death in 2000 (not withdrawn) |
| Dam et al6 (2008) | Woman | 63 | Pantoprazole | 3 d | ANA+ | ANA−, SSA+, SSB−, histone− | Resolution in 4 wk |
| Mankia et al6 (2010) | Woman | 60 | Omeprazole | 4 mo | NA | ANA+, SSA+, SSB−, dsDNA+, histone− | Resolution in 1 mo |
| Present case | Woman | 74 | Esomeprazole | 2 wk | ANA+, SSA+, SSB+, RF+ | ANA+, SSA+, SSB+ | Resolution in 8 wk |
Abbreviations
ANA antinuclear antibody; dsDNA double-stranded anti-DNA antibody; histone antihistone antibody; NA not available; RF rheumatoid factor; SSA anti-Ro/SSA antibody; SSB anti-La/SSB antibody.
The suspect medication must be withdrawn in order to achieve resolution of the symptoms; topical or oral corticosteroids can be used as coadjuvant therapy to speed up recovery. Without withdrawal of the medication, cure is not possible, as shown in previous cases where lesions persisted despite treatment with oral corticosteroids or hydroxychloroquine.6
Our patient was taking a vitamin complex concomitantly with esomeprazole; however, this medication seems a highly unlikely culprit, since there have been no previous reports of cases of lupus erythematosus caused by this agent. Nor can we rule out an episode of idiopathic SCLE, although the apparently clear temporal relationship with the medication and the absence of previous sun exposure and of other characteristic symptoms do not seem to support this possibility as a first option.
To conclude, we present the first case of SCLE induced by esomeprazole. Similarly, we observed positive anti-SSA/Ro and anti-SSB/La antibody titers before the onset of symptoms, suggesting the need for individual predisposition before the condition can develop.
Please cite this article as: Alcántara-González J, et al. Lupues eritematoso cutáneo subagudo inducido por esomeprazol. Actas Dermosifiliogr. 2011;102:640-2.