The aim of these reviews is to describe the reasons for performing skin biopsy, to provide indications for the choice of area to be biopsied and the preparation of the sample, and to summarize the various complications of dermatologic surgery. In addition, we present a guide for selecting the biopsy technique based on the suspected diagnosis and on the area to be biopsied. Finally, the various artifacts that can complicate interpretation of results are described, together with the methods used to prevent their appearance insofar as is possible. The aim of this guide is to improve the diagnostic yield of biopsies and to highlight the importance of a correct clinical–histological correlation.
En estas revisiones se pretende abarcar las diversas funciones de la biopsia cutánea, ciertas nociones básicas acerca de la elección del área a biopsiar y de la forma de procesar la muestra, así como las diversas complicaciones de la cirugía dermatológica de una manera breve. Además, se ofrece una guía del método a elegir para la realización de la biopsia en función tanto del diagnóstico de sospecha, como de algunas localizaciones que ofrecen mayores dificultades. Por último se analizan diversos artefactos que pueden dificultar la interpretación de las lesiones ofreciendo pautas para evitarlos en lo posible. Con esta guía básica pretendemos mejorar la rentabilidad de la biopsia y resaltar la importancia de realizar una correcta correlación clínico-histológica.
The skin is easy to examine clinically and it is also easily accessible for carrying out small surgical procedures, which must nonetheless be done thoughtfully, not mechanically.1 Biopsy procedures are a key step in medical diagnosis, particularly in dermatology because valuable histopathologic information derives from samples that are very readily obtained.1 In the hands of a trained dermatopathologist skin biopsy becomes a valuable tool, and often a simple one, that can facilitate the accurate diagnosis and treatment of diverse dermatoses, particularly tumors. Although most skin biopsies are of good quality, diagnostic challenges arise when an inadequate sample is taken. Therefore, obtaining an adequate biopsy specimen is a complex process involving steps that must be followed carefully, starting with selecting the most appropriate biopsy technique, followed by proper preparation and handling of instruments; the process culminates with a competent dermatopathologist's examination of the tissue under a microscope.2
The clinical dermatologist must have clearly established indications for the procedure, fully explain the intervention to the patient, obtain informed consent, and finally take a tissue specimen that is representative. Clinicians often submit specimens that are too small,3 however, or that have superficial defects due to electrocoagulation or inappropriate use of forceps, or that show signs of drying before they were placed in a fixing solution.
The clinical dermatologist also often chooses an inappropriate site or technique, or may fail to provide the pathologist with even minimal clinical information, making diagnosis difficult.3,4
Another important problem that is often underestimated is the clinician's lack of experience in interpreting histopathologic findings, particularly in inflammatory skin diseases.5 The nature of dermatopathologic nomenclature, and the fact that dermatoses can have multiple names, can also lead to misunderstandings between the clinician and the dermatopathologist. A solid understanding of dermatopathology is therefore necessary if clinicians are to fully take advantage of the possibilities that skin biopsy offers. One study of biopsies of inflammatory lesions taken by dermatologists and other specialists found that the dermatologists’ specimens more often yielded information that led to a specific diagnosis (77% of cases vs 41% for nondermatologists) and that the sites the dermatologists sampled were more appropriate.6
Some US dermatologists have used extenders—other physicians or nurses—to perform biopsies; these dermatologists report that such assistants have a certain tendency to submit ever smaller specimens of inadequate depth for the suspected diagnosis.3 Dermatologists themselves, therefore, should do these procedures in the interest of avoiding diagnostic and therapeutic delays.6
Bearing these problems in mind, and in spite of the lack of consensus-based practice guidelines to help with deciding what size of specimen to take or skin biopsy technique to choose,7 we will summarize the principles and concepts which are important from the perspective of the dermatopathologist and which we believe should be taken into consideration when a skin biopsy is performed. In this first paper we will cover the following points:
- 1.
Functions of skin biopsy: the role of clinical information and technical aspects of the procedure
- 2.
The biopsy: processing the specimen
- 3.
Complications of dermatologic surgery
- 4.
Selecting the biopsy site
Skin biopsy is performed mainly to assist in the accurate diagnosis of a skin disease. For the diagnosis of skin tumors, biopsy provides the best information; biopsy can also help with prognosis if the tumor proves malignant (by showing Breslow depth in melanoma) and can orient treatment, for example in relation to whether or not tumor-free margins are observed on inspection of the specimen (Fig. 1). Biopsy information is also useful in inflammatory dermatoses, allowing several clinical diagnoses to be weighed and finally confirmed or ruled out.
The specimen may be studied by means of conventional staining techniques, direct immunofluorescence, electron microscopy, immunohistochemical staining, tissue culture, polymerase chain reaction techniques, or fluorescence in situ hybridization.8
Skin biopsies are also taken for legal reasons, such as when a dermatopathologic diagnosis is needed to support clinical suspicion,9 and the procedure may also strengthen good physician–patient relations by re-establishing confidence that a correct diagnosis has been made.
Finally, skin biopsies are sometimes required for monitoring purposes, to obtain objective evidence of clinical course, response to treatment, and possible side effects.
Clinical InformationProviding the dermatopathologist with sufficient clinical information to work with might seem obvious, but unfortunately the necessary details are not always communicated. Given that time pressures rule in most clinical settings, too little information is often sent to the laboratory with the specimen.
Lack of detail may be of little importance if the patient has seborrheic keratosis or if an intradermal nevus has been removed for cosmetic reasons. However, the situation is quite different when inflammatory dermatoses or rare neoplastic lesions are involved.6 In such cases differential diagnosis demands the careful consideration of correlations between clinical and histopathologic data; hence the dermatopathologist will need to know the patient's age and sex, the biopsy site, the clinical presentation and time course, the signs and symptoms, and the various local or systemic treatments the patient was using or had used in the past.4
In certain situations, knowledge of earlier biopsy findings might also be useful: this is particularly true in cases of recurrent or persisting melanocytic nevi or other skin tumors that have not been fully excised. Previous biopsy findings can also reveal histologic changes (eg, psoriasis vulgaris, pityriasis rubra pilaris, seborrheic dermatitis, atopic dermatitis, or mycosis fungoides) that prove indispensable for orienting a treatment approach for generalized exanthems (erythroderma).
Some events in the patient's medical history, such as having received a transplant, must be reported, however irrelevant they might seem, because they are associated with certain dermatoses.
Photographs of the lesions may also prove useful, particularly when the tissue specimen is sent for analysis to an external laboratory.10
Technical Aspects of Biopsy ProceduresKnowledge of certain basic principles of dermatopathology are essential for selecting the best biopsy site and technique.
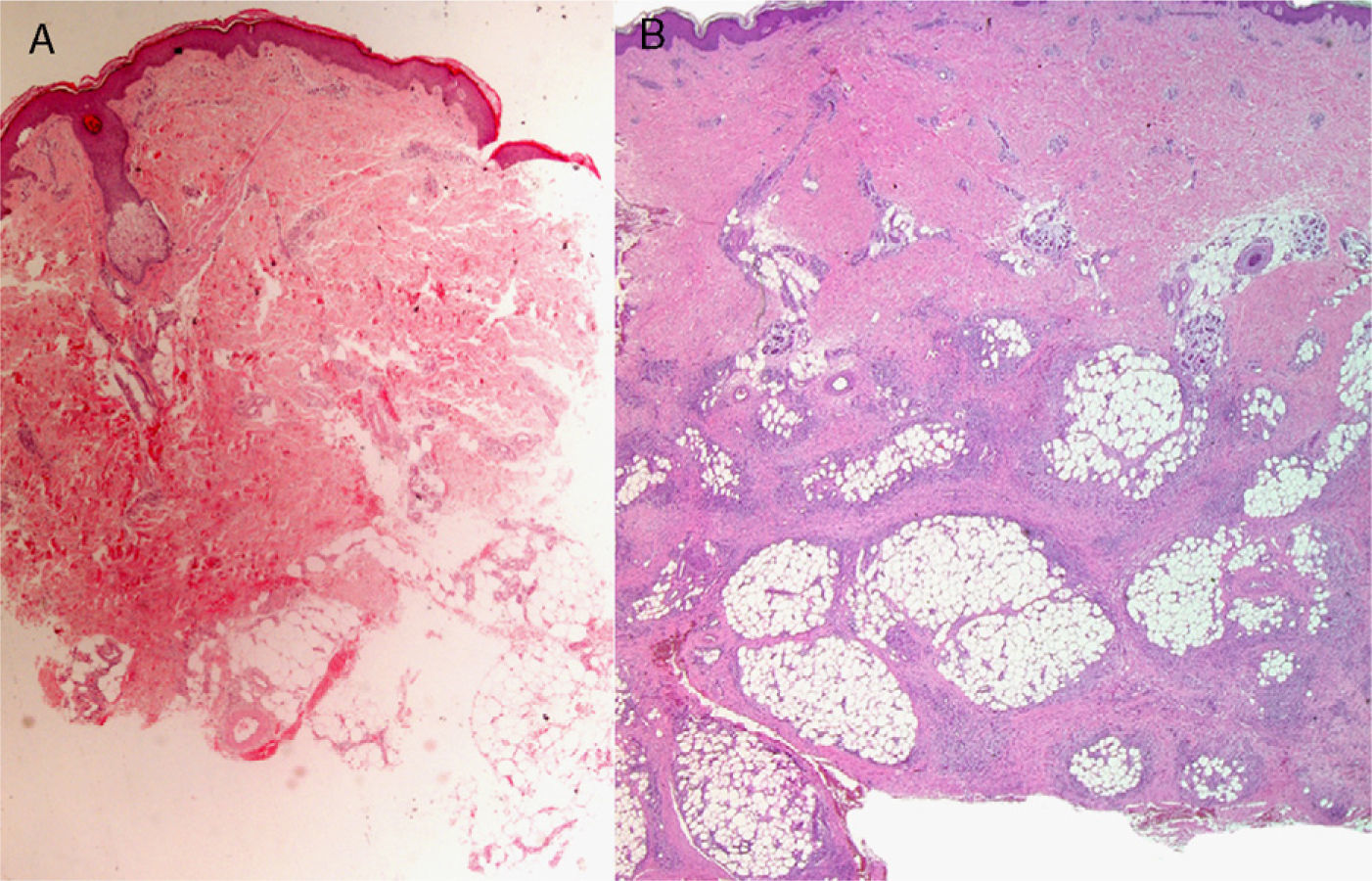
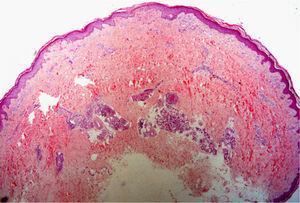
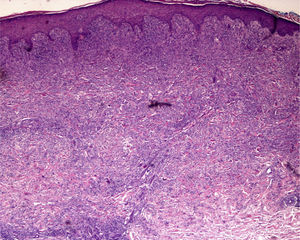
The level of the lesion in the skin must be known (involvement of the epidermis, dermis or subcutaneous tissue) and the specific characteristics of skin at the biopsy site must be understood (Table 1). These considerations are not trivial. For example, dermatopathologists often receive superficial biopsy specimens that contain only the stratum corneum, taken from the palms or soles, where that layer is of greater thickness. Likewise, scalp biopsies often fail to include subcutaneous tissue (making assessment of the hair bulb impossible), and sometimes only the dermis of the lower limbs might have been biopsied in patients with clinical suspicion of panniculitis (Fig. 2).3
Sites of Common Skin Diseases.
| Epidermis and papillary dermis | Melanocytic nevus, age spots, seborrheic keratosis, fibroepithelial polyps, common wartSuperficial basal cell carcinoma, melanoma in situ, mycosis fungoides, actinic keratosis, Paget disease (mammary and extramammary)Contact dermatitis (allergic and irritant), atopic dermatitis, seborrheic dermatitis, plaque psoriasis, scabies, lichen ruber planus, Gibert pityriasis rosacea, vesiculobullous dermatoses |
| Papillary and reticular dermis | Melanocytic nevus, neurofibroma, hemangioma, glomangioma/glomus tumor, sebaceous nevi (hamartomas of the sebaceous follicle), follicular cystsBasal cell carcinoma (solid, sclerodermiform), melanoma, squamous cell carcinomaPhotoallergic dermatitis, phototoxic dermatitis: polymorphic light eruption, scleroderma, morphea, scabietic nodules, leukocytoclastic vasculitis, cutaneous lupus erythematosus, urticaria, granuloma annulare |
| Reticular dermis and subcutaneous tissue | Blue nevus, lipoma, dermatofibroma, epidermoid or trichilemmal cystsMelanoma, cutaneous lymphoma, dermatofibrosarcoma protuberans, metastasis (melanoma, breast cancer, etc.)Panniculitis, sarcoidosis, rheumatoid nodules, nodular vasculitis, polyarteritis nodosa, thrombophlebitis, granuloma annulare |
A, Hematoxylin–eosin, original magnification ×20. Suspected diagnosis, erythema nodosum. Slight perivascular lymphocytic infiltrate. Adipose tissue is scarce and a specific diagnosis cannot be made. B, Hematoxylin–eosin, original magnification ×40. Advanced erythema nodosum with septal panniculitis, granulomatous inflammation, and fibroplasia.
Various skin biopsy techniques are available, as follows:
- 1.
Tangential cut, with scissors.
- 2.
Curettage, with a spoon-shaped curette.
- 3.
Shave biopsy.
- 4.
Punch biopsy, with a circular blade.
- 5.
Elliptical biopsy, which may be either incisional or excisional, according to whether the lesion is partially or completely removed.
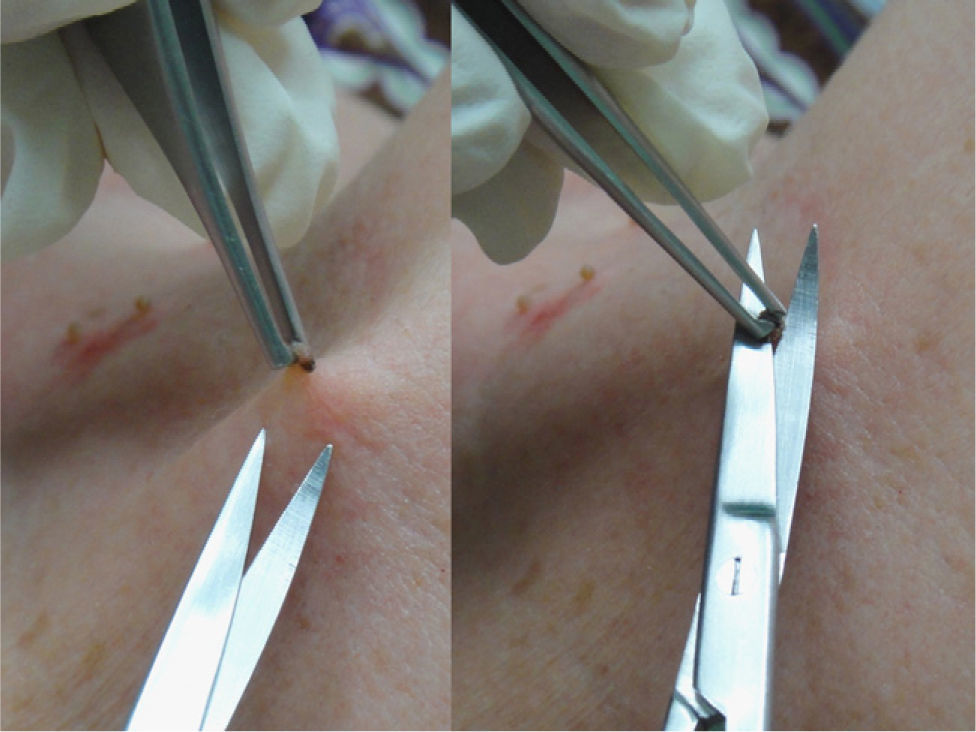
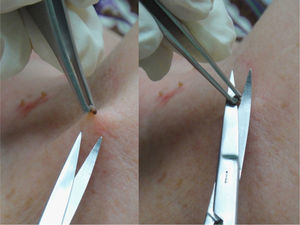
This procedure is optimal for removing superficial lesions, especially pedunculated ones such as pendulous fibromas (polyps, acrochordons, or fibroepithelial papillomas) or seborrheic keratoses (Fig. 3). A local anesthetic is rarely necessary and the wound is usually confined to the papillary dermis.
CurettageCurettage can be useful in dermatology for removing superficial lesions confined to the epidermis. Examples are seborrheic dermatoses, epidermal nevi, common warts, molluscum contagiosum, actinic keratoses, and superficial basal cell epitheliomas. When many small lesions are present, curettage may provide an adequate sample for dermatopathologic diagnosis, although the fragmentation of the specimen or incomplete excision of a tumor may open up questions of malpractice later on.
This technique can be used after application of a cryoanesthetic spray (such as ethyl chloride or liquid nitrogen) or a local anesthetic. The lesion should be grasped firmly between the thumb and index finger with enough pressure to allow a level cut to be made. If the operator cuts too deeply, below the papillary dermis, the incision may leave a scar.11 Essential for executing the technique properly is to hold the lesion firmly enough with one hand to immobilize it and to work deftly with the operating hand. The curette should preferably be held like a pen and rotated with the fingers (the “fountain-pen” technique) or can be grasped with the fingers against the palm of the hand so that the metacarpophalangeal joints can move the blade (the “potato-peeler” technique—in fact, practicing with a potato can help in adjusting the depth of the slice).12
Curettage is contraindicated when a melanocytic lesion is suspected or in any neoplastic lesion of uncertain diagnosis.
Shave BiopsyA fine shaving-like motion is made tangential to the surface of the lesion,13 using either a blade (mounted or not on a scalpel handle) or a disposable curette.
To avoid tissue shrinkage on submerging the specimen in a formol solution, which can make interpretation difficult, place it on a piece of filter paper before fixing.
A shave biopsy is indicated for superficial lesions that are usually exophytic, and excellent cosmetic results can be obtained14 with no need for sutures.
This technique should not be used in inflammatory dermatoses and it is formally contraindicated when melanoma is suspected.
Punch BiopsyCircular blades for punch biopsies are available in diameters from 2 to 8mm. Small diameters (eg, 2mm), which are used only exceptionally, are reserved for cosmetically sensitive sites such as the face. A punch of 4mm is usually large enough.15 Smaller sizes are challenging to the diagnosing dermatopathologist, leading some authors to suggest punches of 6mm, particularly if complementary microbiologic procedures and/or direct immunofluorescence are foreseen, as these will require division of the material.8,11
Punch biopsy is typically performed under local anesthetic; the skin to be biopsied is pinched between two fingers, parallel to the skin tension lines, and the punch is applied perpendicularly. When the punch is rotated, the blade cuts a cylindrical specimen, which can then be pulled up out of the skin, though scissors may be required to separate it from the base. The resulting wound can be sutured if necessary, although the wound may sometimes be left to heal by secondary intention, especially when a small diameter punch has been used.
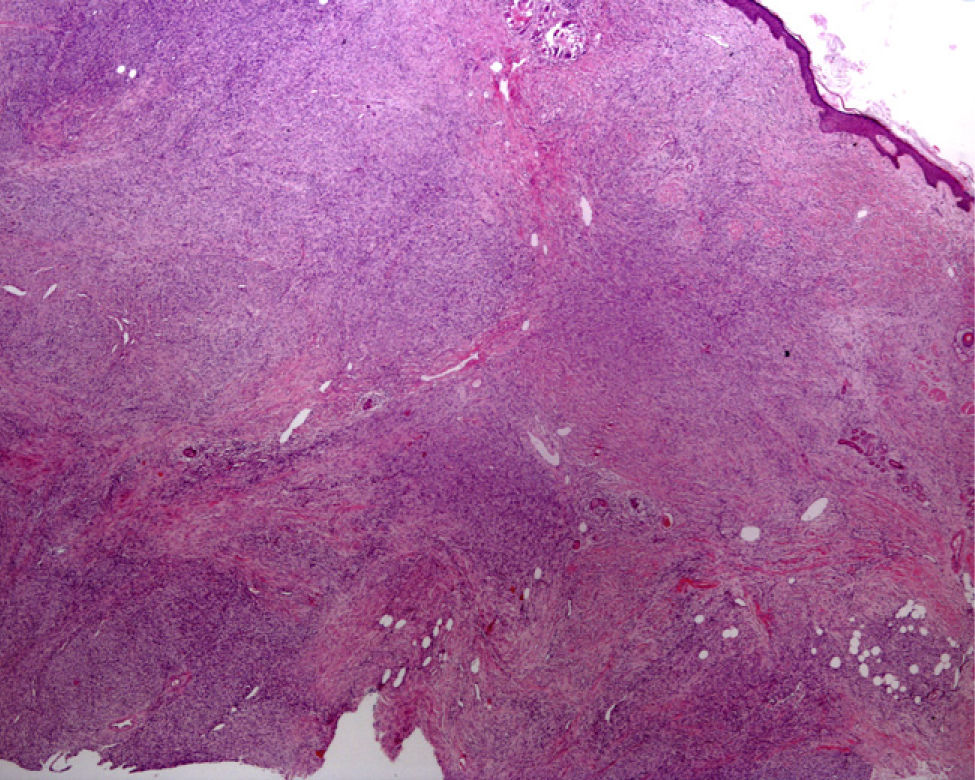
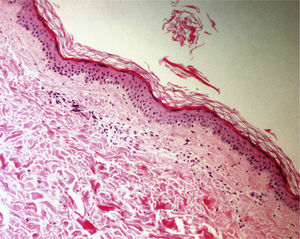
Elliptical BiopsyThe main reason for choosing to perform an elliptical biopsy is to fully excise a tumor, whether benign or malignant. This procedure is also highly useful in non-neoplastic dermatoses such as panniculitis, scarring alopecia, and vasculitis (especially in conditions affecting larger vessels, such as polyarteritis nodosa or thrombophlebitis, in which an excessively superficial biopsy could lead to diagnostic error)16 (Fig. 4). An elliptical biopsy is also called for when ulceration is present and it will be useful to compare healthy and diseased skin.
Hematoxylin–eosin, original magnification ×20. Epidermal and dermal necrosis with thrombi and parietal hyalinosis, findings compatible with a diagnosis of livedoid vasculopathy. Vessels at the dermal–hypodermal junction show foci of segmental fibrinoid necrosis indicating a diagnosis of polyarteritis nodosa.
The endpoints of the incision should be noted on the skin with a sterile marker. After injection of local anesthetic into the area, an incision is made perpendicular to the surface and deep enough to reach subcutaneous tissue.17 The angle between the first and second incision should not exceed 30° and the width between the 2 cuts should be no more than a third the length in the interest of preventing excessive tension once the wound is closed. The incisions should follow the skin tension lines and aesthetic units should be respected.18 Other than taking care to follow these basic principles, the operator should consult more specialized literature for information on such surgical techniques as flap and graft repairs of biopsy sites.
The Biopsy: Processing the SpecimenThe tissue sample should be removed carefully with forceps or a hypodermic needle to avoid damage. Tissue that is to be stained with hematoxylin–eosin should be fixed in a 10% formol solution (4% formaldehyde in water) for about 24hours, though the time required can vary according to the thickness of the specimen.
If possible, use transparent containers for transport so that the contents can be seen easily.
Ideally the formol-to-volume ratio should be 20:1, or at least 5:11; therefore, a variety of collection containers must be on hand in the clinic. To avoid confusion, each biopsy should be placed in a separate, properly labeled receptacle.
Tumor excision biopsies must be inspected to ensure they are complete and that the borders of the lesion are included. One side of the prepared specimen should be marked, usually by placing a suture to designate the cephalad border, trying to leave the strand loose enough so that the specimen remains undamaged when the suture is removed; otherwise evaluation may be difficult. Once the suture is removed in the laboratory, the specimen is marked again and cut into cross-sections. If there is residual lesion, it will be possible to verify its exact location.
When material is to be used for immunofluorescence, the specimen must be divided into 2 parts. One will be processed as already described, by fixing it in formol. The other portion will be wrapped in gauze moistened with normal saline solution and placed in an appropriate receptacle, which does not need to be filled with saline since it is only necessary to keep the tissue moist. If transporting the specimen to an external laboratory will take more than 24hours, it should be placed inside a larger, isothermal carrier packed with dry ice. The specimen might also be placed in Michel's transport medium (an ammonium sulfide, N-ethylmaleimide, and magnesium sulfate solution), which will preserve the specimen well for as long as 10 days.19
For diagnosis on the basis of frozen specimen sections, as in Mohs micrographic surgery, the tissue is preserved in a special medium, usually an optimum cutting temperature (OCT) medium composed of water-soluble glycols and resins; the specimen is embedded and then frozen for cryostat sectioning.
A specimen to be studied by electron microscopy must be placed for 2hours in a 0.5% Karnovsky solution (0.5% glutaraldehyde, 2% paraformaldehyde in a 0.2M cacodylate buffer at a pH of 7.3) and later fixed in osmium tetroxide in the same buffer solution.20 In this technique, the specimen must be dried before cutting into thin sections for staining (eg, with uranyl acetate); this treatment is useful, for example, for immune phenotyping of amyloidosis in abdominal fat biopsies.20
When obtaining specimens for microbiology, a sterile receptacle should be used and the tissue wrapped in gauze soaked in normal saline (ie, a nonbacteriostatic solution). Samples for viral culture can be transported in a liquid medium. Contact the microbiology laboratory for specific instructions if in doubt.
Patient Considerations and Complications of Dermatopathologic SurgeryAs a well informed patient is more cooperative, the dermatologist should explain the reasons for the biopsy, the surgical technique that will be used, and the possible side effects.
Dermatologic surgery does not usually lead to life-threatening complications, although anaphylactic reactions or arrhythmias may occur; therefore, it is important to be ready to perform basic resuscitation maneuvers if need be.21
Most complications involve bleeding, infection and unsightly scars or changes in pigmentation. In taking the medical history, therefore, it is important to note predisposing factors such as malnutrition, advanced age, metabolic disease or genodermatoses, medication, smoking or alcohol intake, or previous therapeutic procedures.21
The possibility of doing a shave biopsy should be discussed, particularly if a melanocytic tumor is suspected, considering the cosmetic advantages of this technique but also the medical disadvantages.
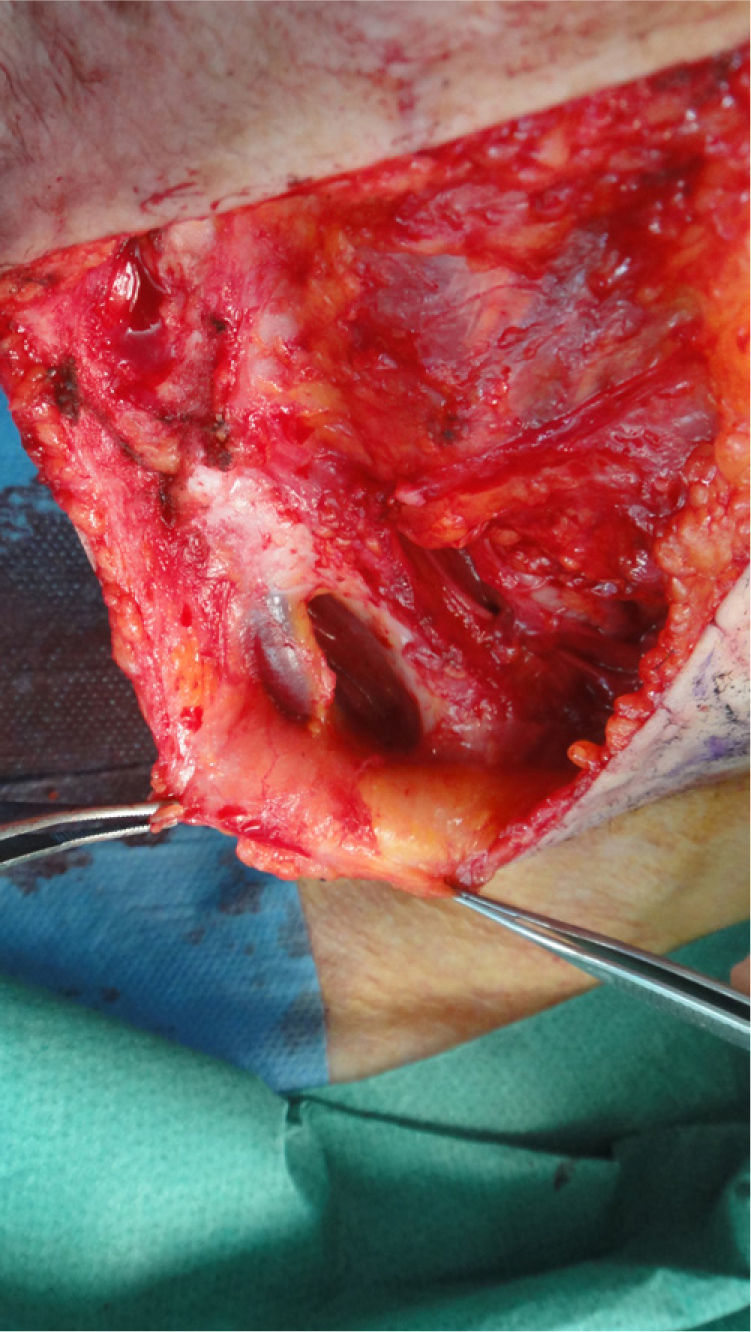
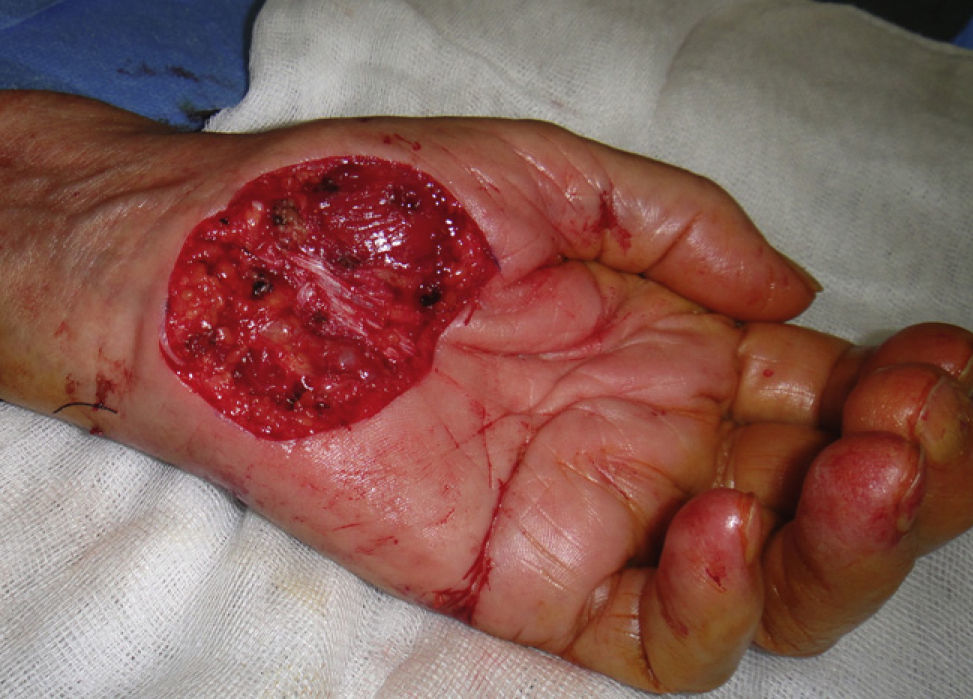
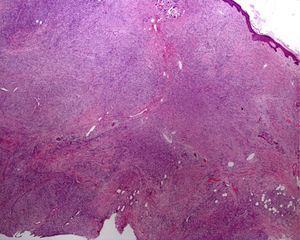
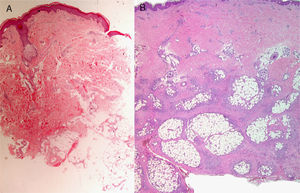
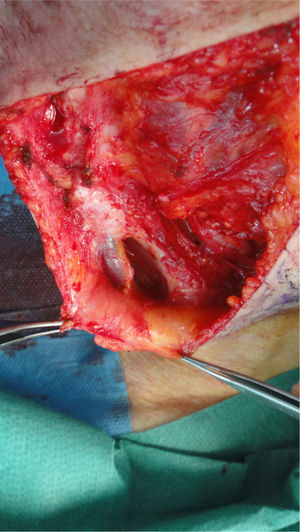
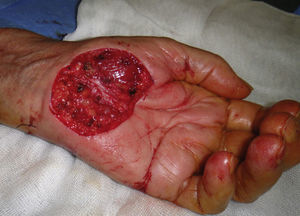
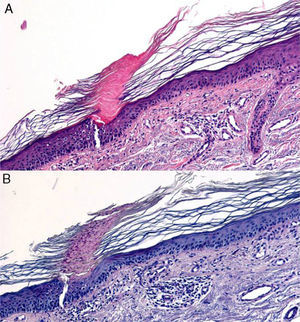
The possibility of injuring delicate anatomical structures, such as large vessels, nerves or articular capsules might constitute a relative contraindication.14 Particular care must be taken when working near large pulsatile vessels in the trunk or head and neck (Fig. 5). The presence of structures deep to the biopsy site (such as neurovascular bundles and even spinal fluid) must also be taken into consideration (Fig. 6). Thorough knowledge of the anatomy of the biopsy site is therefore essential.21
Alcohol reduces skin flora by 75% within a minute of application and is usually adequate for disinfection, but it is mainly effective against gram-positive microorganisms. A povidone–iodine solution's effect is slower than that of alcohol,1 but its antibacterial spectrum is broader, as it acts against some gram-negative microorganisms as well as gram-positive ones. Chlorhexidine, which is also effective against both types, has a rapid onset of effect that lasts for several hours. This disinfectant should not be used near the eye to avoid irritation, but it is the most effective antiseptic agent both for prevention and for application on the surgical field.22
Several antiseptics can be used in combination.
AnesthesiaThe biopsy site can be adequately anesthetized with an infiltration anesthetic such as lidocaine, to which epinephrine (1:100000) can be added to prolong the effect and curb bleeding; mepivacaine and bupivacaine are possible alternatives. The local anesthetic can also be combined with the use of a cryogen or topical anesthetic, for example, EMLA cream (an eutectic mixture of prilocaine and lidocaine) or 4% lidocaine cream.
No product is risk-free and there have been reports of erythema, contact urticaria, irritative dermatitis, and more rarely, methemoglobinemia and purpura in association with EMLA cream.23
Prophylactic Antibiotic TherapyWound infection is one of the factors that can delay healing. Presurgical antibiotic therapy to prevent endocarditis is not recommended in patients undergoing surgery on clean, noninflamed skin under any circumstances.22 However, prophylaxis is recommended for high-risk patients (those with prosthetic valves or a history of endocarditis or complex cyanotic congenital heart disease) when surgery will affect mucosas, central areas of the face, or genitalia; prophylaxis is also recommended before Mohs micrographic surgery involving evaluation of fresh tissue.22
Prophylaxis should cover the usual skin flora, mainly gram-positive microorganisms (staphylococci and streptococci). Oral cephalexin (2g before surgery and 500mg 6hours later if indicated) is the treatment of choice, although it is also possible to use amoxicillin (2g orally) or cloxacillin (2g orally). In allergic patients, use erythromycin (1g orally before surgery and 500mg afterwards), azithromycin (500mg orally), clarithromycin (500mg orally), or clindamycin (600mg orally).22 The presurgical dose should be given 30–60minutes beforehand.22 The decision to prescribe prophylaxis for patients who have recently (within the past 2 years) been implanted with a prosthesis should be made on a case-by-case basis after discussion between the dermatologist and surgeon.22
Similar infection rates have been reported in patients using bacitracin or mupirocin cream on the biopsy site and those using petroleum jelly.24
If oral antibiotics are prescribed, they should be reserved for cases in which there will be flap or graft repair in the area around the nose, the wound closure will be subject to tension, or the surgeon will be acting on infected lesions21 or on sites at higher risk of infection or where functional consequences could be significant (eg, the hand).22
Anticoagulant and Antiplatelet TherapyImportant factors to take into account are whether the patient is taking oral anticoagulants or has a systemic disease that increases the risk of coagulation disorders, thrombocytopenia, or immune system compromise. The presence of diabetic angiopathy, lower limb edema or chronic venous insufficiency should also be taken into account, particularly when the biopsy site is on the lower extremities.
In a recent review, Bassas et al.25 concluded that warfarin therapy should not be suspended before dermatologic surgery, although the surgical technique should be planned carefully, with strict hemostatic measures, and there should be adequate postoperative follow-up. An international normalized ratio between 2.5 and 3 would be acceptable in patients treated with warfarin who are candidates for dermatologic surgery.25,26
Less is known about the use of antiplatelet therapy (with acetylsalicylic acid or other cyclooxygenase inhibitors). Although it seems that combined use of clopidogrel and acetylsalicylic acid increases the risk of complications in Mohs surgery,27 suspending therapy does not seem to be necessary.25,28
Selecting the Biopsy SiteInflammatory skin diseases have successive phases. For that reason it is not always possible to perform biopsies during an acute episode, when the most information will be obtained because the later changes present in advanced lesions are often nonspecific and do not contribute to the diagnosis (and may even make it more difficult). The patient does not always seek care as soon as lesions appear, however, and biopsy is sometimes delayed.6
Certain biopsies can be avoided, such when cutaneous mastocytosis is suspected after Darier's sign is elicited; in this disease, degranulating mast cells are barely discernible even with immunohistochemistry.
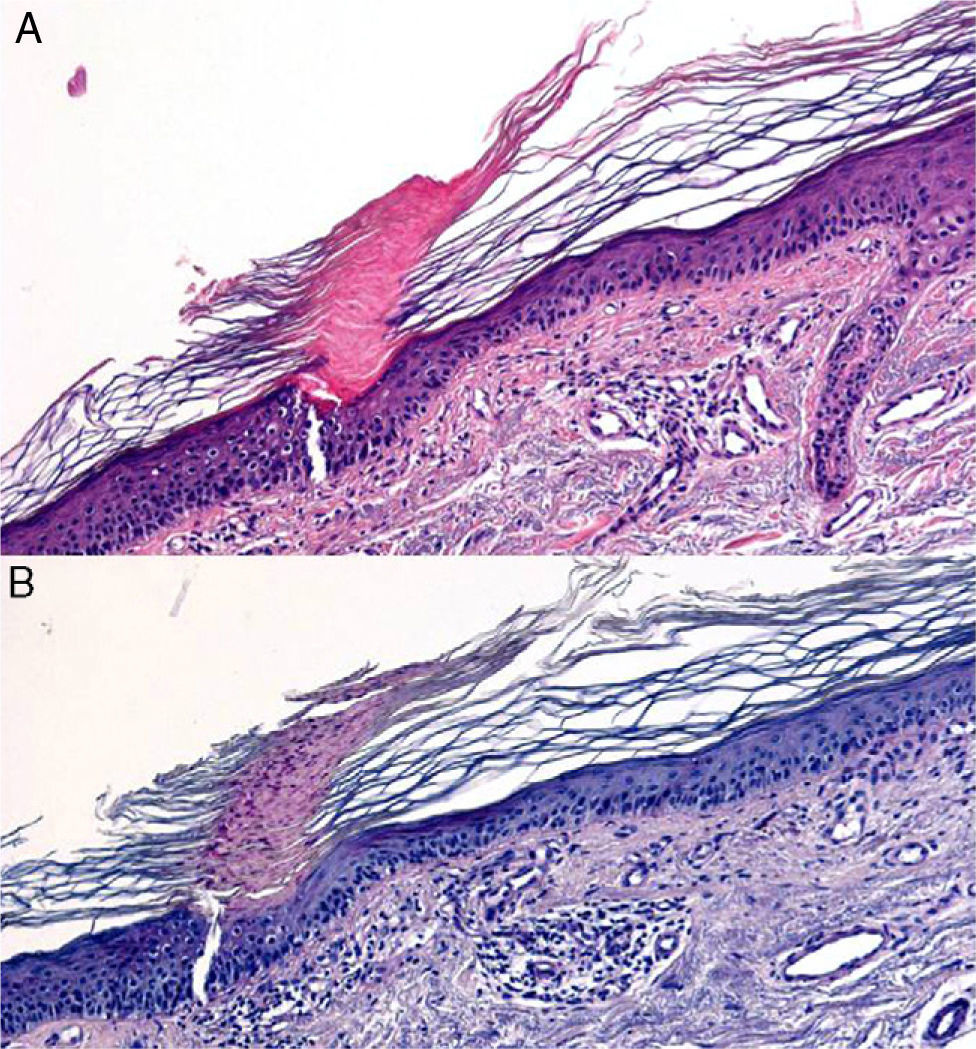
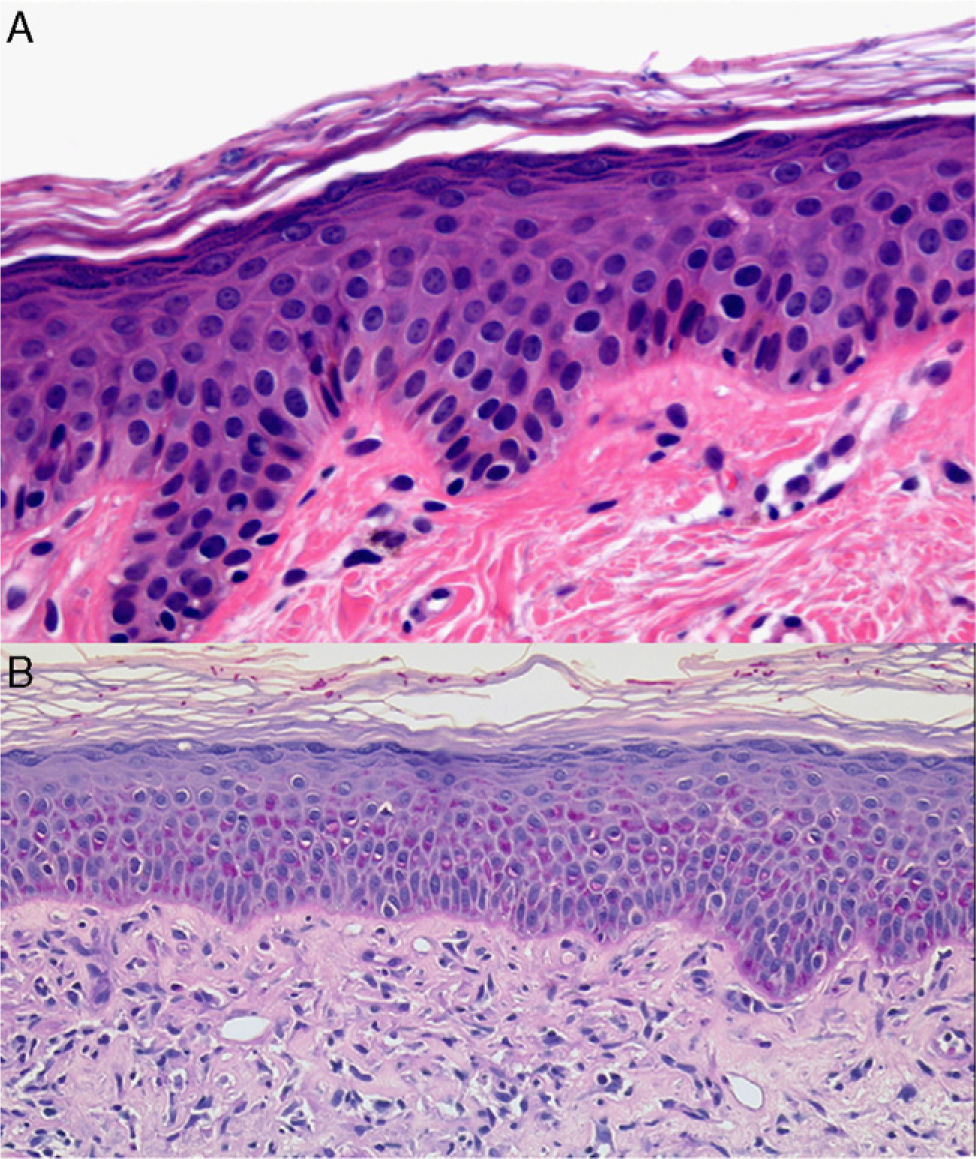
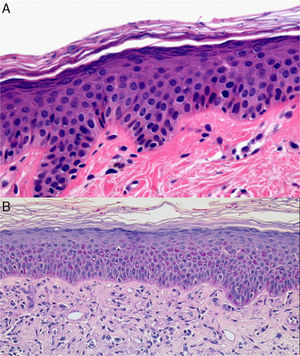
Bullous dermatoses (bullous pemphigoid, pemphigus vulgaris, dermatitis herpetiformis, etc) often begin with an urticarial rash that has a histologic pattern of eosinophilic spongiosis affecting the epidermis and papillary dermis. This pattern can also be seen in other conditions: allergic contact eczema, nummular eczema, dermatitis medicamentosa, bites from insects and other arthropods, scabies, urticaria, incontinentia pigmenti, and Leiner disease (toxic erythema).29,30 Bear in mind that after the site has been treated with corticosteroid creams the eosinophil count will decrease and diagnosis may become more difficult. When diagnosing blistering diseases, examination of the site of cleavage can be useful. If biopsy has been delayed, the only observations may be erosion and ulceration, or repair processes or signs of bacterial colonization. Histologic findings vary according to the phase when the biopsy is performed in certain diseases (leukocytoclastic vasculitis, juvenile xanthogranuloma [Fig. 7] or pityriasis lichenoides et varioliformis acuta) and may initially be nonspecific (Fig. 8). Several biopsies may therefore be required before a precise diagnosis can be made. For annular lesions (eg, granuloma annulare, dermatomycosis, erythema chronicum migrans, erythema annulare centrifugum, cutaneous lupus erythematosus, porokeratosis and others), tissue must be sampled from the active border (Fig. 9).
From either a clinical or histologic perspective, an etiologic diagnosis is hard to achieve in erythroderma, also known as exfoliative dermatitis. When taking the medical history, questions about medication and substance use must be included as well as investigation of previous skin diseases. A thorough physical examination should include palpation to detect masses, organomegaly, and lymphadenopathy. Even so, several biopsy procedures are necessary for diagnosis in over half the cases, and the diagnosis usually rests on a combination of histopathologic and clinical factors.31
Whenever possible, the operator should take samples from several representative sites on the trunk or proximal extremities, given that skin on the distal lower extremities nearly always shows a degree of inflammatory infiltrate and changes related to superimposed stasis dermatitis; moreover, surgical wounds in distal areas will heal more slowly.32
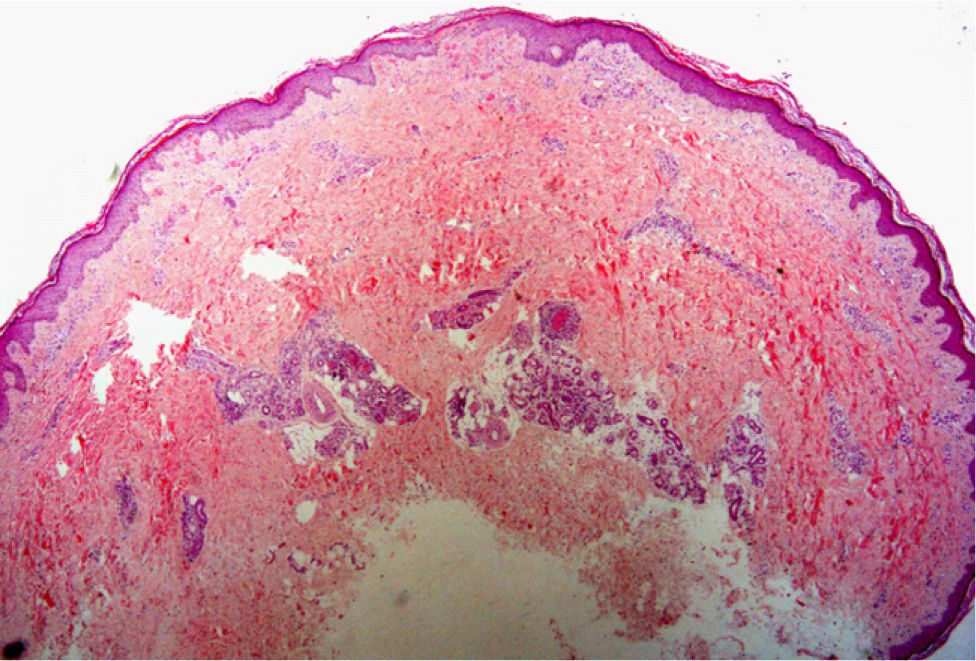
For the accurate diagnosis of panniculitis lesions, biopsy plays a crucial role. Deep incisional biopsies are required to ensure taking an adequate sample of adipose tissue. If the operator chooses to do a punch biopsy, the blade should be at least 6mm in diameter. To interpret the findings, it will be important to know the location or distribution of the infiltrate (predominantly lobular or septal), the predominating cell populations, and the presence or absence of vasculitis.33,34
To study pigmentation changes, particularly postinflammatory hypopigmentation or vitiligo, the operator should take a sample that contains both lesional and healthy skin (for comparison within the same specimen). Specific stains, such as Masson–Fontana stain, and immunohistochemical analysis (eg, with S-100, Melan-A) can help pinpoint the diagnosis. Periodic acid–Schiff (PAS) staining should also be performed to rule out or identify fungal infections (mainly pityriasis versicolor) (Fig. 10). PAS staining can also help identify hyphae and spores when pigmentation changes are present in patients who have been treated with antifungal agents.
A, Hematoxylin–eosin, original magnification ×400. Long and oval-shaped structures, bluish in color, corresponding to Malassezia species, can be seen in the stratum corneum. B, Periodic acid–Schiff stain, original magnification ×40. Hyphae can be observed in the stratum corneum: diagnosis, tinea infection.
The authors declare that they have no conflicts of interest.
Please cite this article as. Llamas-Velasco M. Paredes BE. La biopsia cutánea: bases fundamentales. Parte I. Actas Dermosifiliogr. 2012;103:12-20.