Atopic dermatitis (AD) is a chronic skin disease characterized by outbreaks of pruritic eczematous lesions involving an impaired quality of life for the patient1,2 The origin of AD is multifactorial, with its pathogenesis involving immune dysregulation characterized by an increased cutaneous Th2 lymphocyte response, leading to elevated levels of interleukins (IL): IL-4, IL-5, IL-13, IL-25, and IL-31.3
Up until the appearance of biological therapies with dupilumab—the first monoclonal antibody against IL-4/IL-13—in 2007 the management of moderate-to-severe AD posed a therapeutic challenge due to the lack of effective drugs with a good long-term safety profile.1,2 Since then, other targeted therapies have been approved, among them, tralokinumab (2021), a monoclonal antibody directed against IL-13 that prevents its interaction with the IL-13Rα1/IL-4Rα receptor.3–5 The aim of this study was to evaluate the safety and efficacy profile of tralokinumab in the management of moderate-to-severe AD.
We conducted a descriptive, retrospective study of all patients with cyclosporine-refractory moderate-to-severe AD treated with tralokinumab (1 subcutaneous injection of 600mg followed by 300mg every 2 weeks) for, at least, 16 weeks from June 2022 through February 2023 at a Spanish tertiary referral center. Data were collected from the patients’ electronic health records, including sex, age, dosage, treatment duration, and previous treatments. Effectiveness was evaluated using the SCORing Atopic Dermatitis (SCORAD) and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-ADTM) scales at baseline, and on weeks 16 and 24.6,7
The main outcome variables of the study were the proportion of patients who achieved responses between 0 and 1 on the vIGA-AD scale (vIGA-AD 0-1) 16 and 24 weeks after treatment, and a reduction of, at least, 75% from baseline in the SCORAD scale (SCORAD-75). Secondary variables included a reduction of, at least, 90% from baseline in the SCORAD scale (SCORAD-90), and “total clearance of the disease” or SCORAD of 0 between such weeks. Finally, drug safety was evaluated by collecting all adverse effects (AE) and their suspensions.
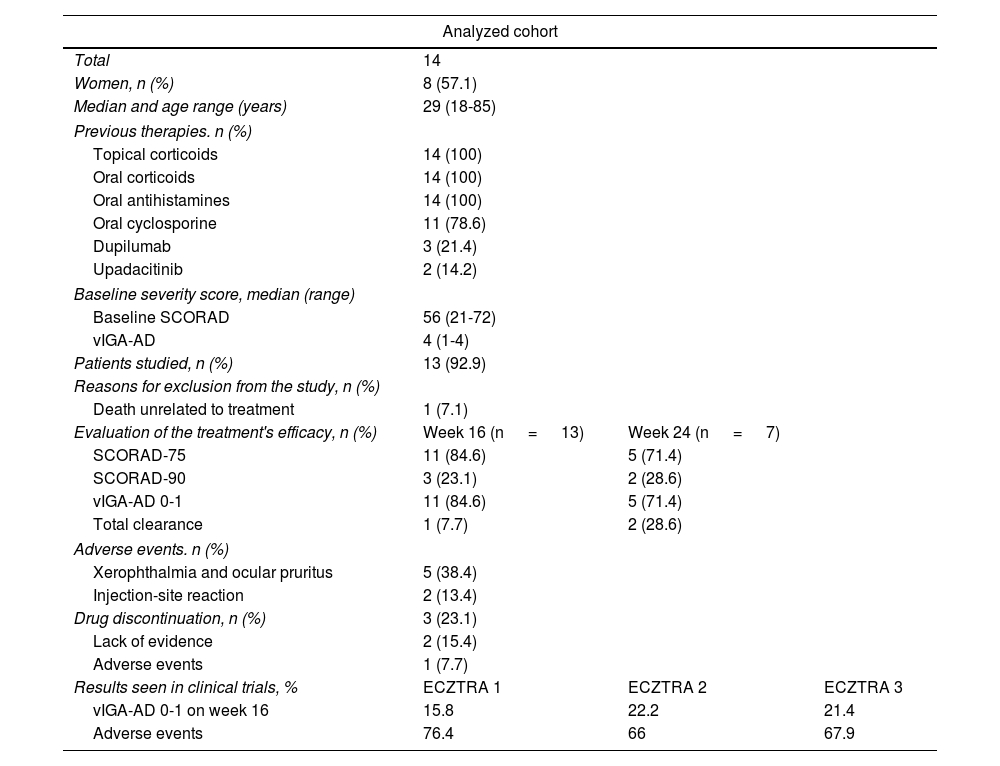
A total of 14 patients were included, whose sociodemographic characteristics and previous therapies are shown in Table 1. The median baseline scores were SCORAD-56 (range, 21-72) and vIGA-AD 4 (range, 1-4). The median treatment duration was 24 weeks (range, 16-32). All patients were evaluated on week 16. However, at the time of the study, only half of the patients had completed a sufficient treatment period including evaluation on week 24 (7/13; 53.8%). One patient was excluded due to death unrelated to the studied disease.
Results of the analyzed cohort and results observed in the pivotal clinical trials ECZTRA 1, ECZTRA 2, and ECZTRA 3.
| Analyzed cohort | |||
|---|---|---|---|
| Total | 14 | ||
| Women, n (%) | 8 (57.1) | ||
| Median and age range (years) | 29 (18-85) | ||
| Previous therapies. n (%) | |||
| Topical corticoids | 14 (100) | ||
| Oral corticoids | 14 (100) | ||
| Oral antihistamines | 14 (100) | ||
| Oral cyclosporine | 11 (78.6) | ||
| Dupilumab | 3 (21.4) | ||
| Upadacitinib | 2 (14.2) | ||
| Baseline severity score, median (range) | |||
| Baseline SCORAD | 56 (21-72) | ||
| vIGA-AD | 4 (1-4) | ||
| Patients studied, n (%) | 13 (92.9) | ||
| Reasons for exclusion from the study, n (%) | |||
| Death unrelated to treatment | 1 (7.1) | ||
| Evaluation of the treatment's efficacy, n (%) | Week 16 (n = 13) | Week 24 (n = 7) | |
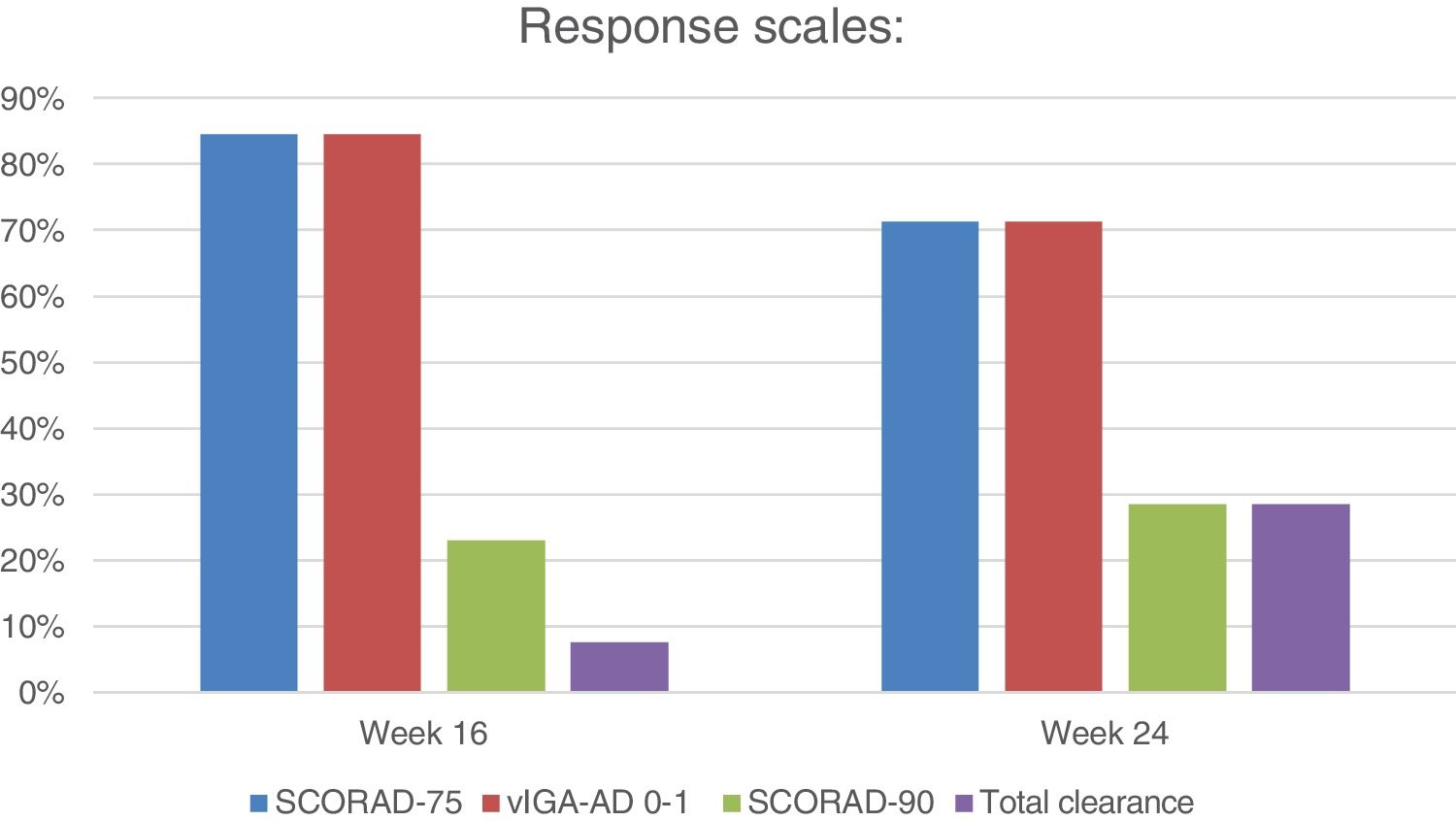
| SCORAD-75 | 11 (84.6) | 5 (71.4) | |
| SCORAD-90 | 3 (23.1) | 2 (28.6) | |
| vIGA-AD 0-1 | 11 (84.6) | 5 (71.4) | |
| Total clearance | 1 (7.7) | 2 (28.6) | |
| Adverse events. n (%) | |||
| Xerophthalmia and ocular pruritus | 5 (38.4) | ||
| Injection-site reaction | 2 (13.4) | ||
| Drug discontinuation, n (%) | 3 (23.1) | ||
| Lack of evidence | 2 (15.4) | ||
| Adverse events | 1 (7.7) | ||
| Results seen in clinical trials, % | ECZTRA 1 | ECZTRA 2 | ECZTRA 3 |
| vIGA-AD 0-1 on week 16 | 15.8 | 22.2 | 21.4 |
| Adverse events | 76.4 | 66 | 67.9 |
Regarding the main variables, on week 16, 11 patients (11/13; 84.6%) achieved a vIGA-AD 0-1 response, and SCORAD-75 (figure 1). On week 24, 5 of 7 evaluated patients (5/7; 71.4%) achieved a vIGA-AD 0-1 response and SCORAD-75. Regarding secondary variables, 3 patients achieved SCORAD-90 on week 16 (3/13; 23.1%) while 2 patients did so on week 24 (2/7; 28.6%). One (1/13; 7.7%) and 2 patients (2/7; 28.6%) achieved total clearance on weeks 16 and 24, respectively. Three patients (3/13; 23.1%) discontinued tralokinumab, 2 of them (2/13; 15.4%) due to inefficacy after a median 7-month therapeutic regimen (range, 5-9), and 1 (1/13; 7.7%) due to an injection-site reaction.
Half of the patients (7/13; 53.8%) experienced some AEs, mostly of mild. Five patients (5/13; 38.4%) reported xerophthalmia and conjunctivitis, which well tolerated with symptomatic measures, while 2 (2/13; 13.4%) had an injection site-related AE.
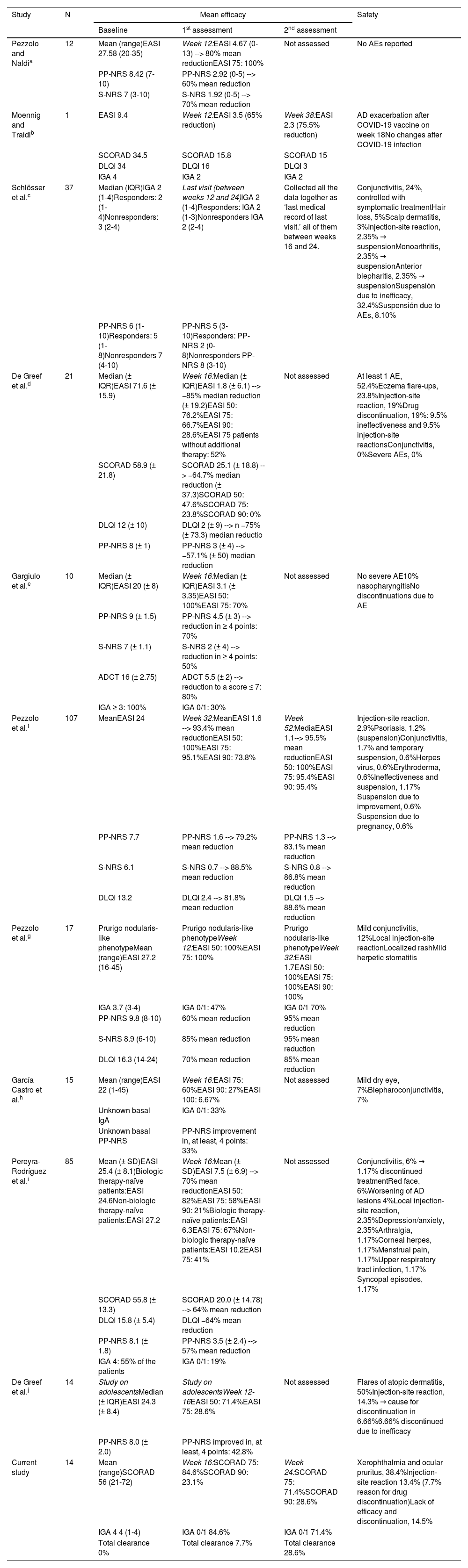
Tralokinumab proved safe and effective to treat moderate-to-severe AD in clinical trials (ECZTRA 1, ECZTRA 2, ECZTRA 3).8,9 There are few long-term studies of its use in the routine clinical practice (Table 2), whose results are comparable to those observed in our study.5–7 Most patients responded within 16 weeks, maintaining response until week 24. Only 15% had to stop tralokinumab due to inefficacy. Tolerance was adequate, and half experienced mainly mild ocular AEs, which were treated symptomatically. Only 1 case required treatment discontinuation following an injection site-related AE.
Safety and efficacy visualized in real-life experience studies of tralokinumab for moderate-to-severe atopic dermatitis published to this date.
| Study | N | Mean efficacy | Safety | ||
|---|---|---|---|---|---|
| Baseline | 1st assessment | 2nd assessment | |||
| Pezzolo and Naldia | 12 | Mean (range)EASI 27.58 (20-35) | Week 12:EASI 4.67 (0-13) --> 80% mean reductionEASI 75: 100% | Not assessed | No AEs reported |
| PP-NRS 8.42 (7-10) | PP-NRS 2.92 (0-5) --> 60% mean reduction | ||||
| S-NRS 7 (3-10) | S-NRS 1.92 (0-5) --> 70% mean reduction | ||||
| Moennig and Traidlb | 1 | EASI 9.4 | Week 12:EASI 3.5 (65% reduction) | Week 38:EASI 2.3 (75.5% reduction) | AD exacerbation after COVID-19 vaccine on week 18No changes after COVID-19 infection |
| SCORAD 34.5 | SCORAD 15.8 | SCORAD 15 | |||
| DLQI 34 | DLQI 16 | DLQI 3 | |||
| IGA 4 | IGA 2 | IGA 2 | |||
| Schlösser et al.c | 37 | Median (IQR)IGA 2 (1-4)Responders: 2 (1-4)Nonresponders: 3 (2-4) | Last visit (between weeks 12 and 24)IGA 2 (1-4)Responders: IGA 2 (1-3)Nonresponders IGA 2 (2-4) | Collected all the data together as ‘last medical record of last visit.’ all of them between weeks 16 and 24. | Conjunctivitis, 24%, controlled with symptomatic treatmentHair loss, 5%Scalp dermatitis, 3%Injection-site reaction, 2.35% → suspensionMonoarthritis, 2.35% → suspensionAnterior blepharitis, 2.35% → suspensionSuspensión due to inefficacy, 32.4%Suspensión due to AEs, 8.10% |
| PP-NRS 6 (1-10)Responders: 5 (1-8)Nonresponders 7 (4-10) | PP-NRS 5 (3-10)Responders: PP-NRS 2 (0-8)Nonresponders PP-NRS 8 (3-10) | ||||
| De Greef et al.d | 21 | Median (± IQR)EASI 71.6 (± 15.9) | Week 16:Median (± IQR)EASI 1.8 (± 6.1) --> −85% median reduction (± 19.2)EASI 50: 76.2%EASI 75: 66.7%EASI 90: 28.6%EASI 75 patients without additional therapy: 52% | Not assessed | At least 1 AE, 52.4%Eczema flare-ups, 23.8%Injection-site reaction, 19%Drug discontinuation, 19%: 9.5% ineffectiveness and 9.5% injection-site reactionsConjunctivitis, 0%Severe AEs, 0% |
| SCORAD 58.9 (± 21.8) | SCORAD 25.1 (± 18.8) --> −64.7% median reduction (± 37.3)SCORAD 50: 47.6%SCORAD 75: 23.8%SCORAD 90: 0% | ||||
| DLQI 12 (± 10) | DLQI 2 (± 9) --> n −75% (± 73.3) median reductio | ||||
| PP-NRS 8 (± 1) | PP-NRS 3 (± 4) --> −57.1% (± 50) median reduction | ||||
| Gargiulo et al.e | 10 | Median (± IQR)EASI 20 (± 8) | Week 16:Median (± IQR)EASI 3.1 (± 3.35)EASI 50: 100%EASI 75: 70% | Not assessed | No severe AE10% nasopharyngitisNo discontinuations due to AE |
| PP-NRS 9 (± 1.5) | PP-NRS 4.5 (± 3) --> reduction in ≥ 4 points: 70% | ||||
| S-NRS 7 (± 1.1) | S-NRS 2 (± 4) --> reduction in ≥ 4 points: 50% | ||||
| ADCT 16 (± 2.75) | ADCT 5.5 (± 2) --> reduction to a score ≤ 7: 80% | ||||
| IGA ≥ 3: 100% | IGA 0/1: 30% | ||||
| Pezzolo et al.f | 107 | MeanEASI 24 | Week 32:MeanEASI 1.6 --> 93.4% mean reductionEASI 50: 100%EASI 75: 95.1%EASI 90: 73.8% | Week 52:MediaEASI 1.1--> 95.5% mean reductionEASI 50: 100%EASI 75: 95.4%EASI 90: 95.4% | Injection-site reaction, 2.9%Psoriasis, 1.2% (suspension)Conjunctivitis, 1.7% and temporary suspension, 0.6%Herpes virus, 0.6%Erythroderma, 0.6%Ineffectiveness and suspension, 1.17% Suspension due to improvement, 0.6% Suspension due to pregnancy, 0.6% |
| PP-NRS 7.7 | PP-NRS 1.6 --> 79.2% mean reduction | PP-NRS 1.3 --> 83.1% mean reduction | |||
| S-NRS 6.1 | S-NRS 0.7 --> 88.5% mean reduction | S-NRS 0.8 --> 86.8% mean reduction | |||
| DLQI 13.2 | DLQI 2.4 --> 81.8% mean reduction | DLQI 1.5 --> 88.6% mean reduction | |||
| Pezzolo et al.g | 17 | Prurigo nodularis-like phenotypeMean (range)EASI 27.2 (16-45) | Prurigo nodularis-like phenotypeWeek 12:EASI 50: 100%EASI 75: 100% | Prurigo nodularis-like phenotypeWeek 32:EASI 1.7EASI 50: 100%EASI 75: 100%EASI 90: 100% | Mild conjunctivitis, 12%Local injection-site reactionLocalized rashMild herpetic stomatitis |
| IGA 3.7 (3-4) | IGA 0/1: 47% | IGA 0/1 70% | |||
| PP-NRS 9.8 (8-10) | 60% mean reduction | 95% mean reduction | |||
| S-NRS 8.9 (6-10) | 85% mean reduction | 95% mean reduction | |||
| DLQI 16.3 (14-24) | 70% mean reduction | 85% mean reduction | |||
| García Castro et al.h | 15 | Mean (range)EASI 22 (1-45) | Week 16:EASI 75: 60%EASI 90: 27%EASI 100: 6.67% | Not assessed | Mild dry eye, 7%Blepharoconjunctivitis, 7% |
| Unknown basal IgA | IGA 0/1: 33% | ||||
| Unknown basal PP-NRS | PP-NRS improvement in, at least, 4 points: 33% | ||||
| Pereyra-Rodríguez et al.i | 85 | Mean (± SD)EASI 25.4 (± 8.1)Biologic therapy-naïve patients:EASI 24.6Non-biologic therapy-naïve patients:EASI 27.2 | Week 16:Mean (± SD)EASI 7.5 (± 6.9) --> 70% mean reductionEASI 50: 82%EASI 75: 58%EASI 90: 21%Biologic therapy-naïve patients:EASI 6.3EASI 75: 67%Non-biologic therapy-naïve patients:EASI 10.2EASI 75: 41% | Not assessed | Conjunctivitis, 6% → 1.17% discontinued treatmentRed face, 6%Worsening of AD lesions 4%Local injection-site reaction, 2.35%Depression/anxiety, 2.35%Arthralgia, 1.17%Corneal herpes, 1.17%Menstrual pain, 1.17%Upper respiratory tract infection, 1.17% Syncopal episodes, 1.17% |
| SCORAD 55.8 (± 13.3) | SCORAD 20.0 (± 14.78) --> 64% mean reduction | ||||
| DLQI 15.8 (± 5.4) | DLQI −64% mean reduction | ||||
| PP-NRS 8.1 (± 1.8) | PP-NRS 3.5 (± 2.4) --> 57% mean reduction | ||||
| IGA 4: 55% of the patients | IGA 0/1: 19% | ||||
| De Greef et al.j | 14 | Study on adolescentsMedian (± IQR)EASI 24.3 (± 8.4) | Study on adolescentsWeek 12-16EASI 50: 71.4%EASI 75: 28.6% | Not assessed | Flares of atopic dermatitis, 50%Injection-site reaction, 14.3% → cause for discontinuation in 6.66%6.66% discontinued due to inefficacy |
| PP-NRS 8.0 (± 2.0) | PP-NRS improved in, at least, 4 points: 42.8% | ||||
| Current study | 14 | Mean (range)SCORAD 56 (21-72) | Week 16:SCORAD 75: 84.6%SCORAD 90: 23.1% | Week 24:SCORAD 75: 71.4%SCORAD 90: 28.6% | Xerophthalmia and ocular pruritus, 38.4%Injection-site reaction 13.4% (7.7% reason for drug discontinuation)Lack of efficacy and discontinuation, 14.5% |
| IGA 4 4 (1-4) | IGA 0/1 84.6% | IGA 0/1 71.4% | |||
| Total clearance 0% | Total clearance 7.7% | Total clearance 28.6% | |||
The above are the different real-world studies of tralokinumab available after conducting a bibliographic search on PubMed using the criteria “Tralokinumab” and “Real life” or “Clinical practice.” Real-world studies whose main objectives were to assess dupilumab-related conjunctivitis after switching to tralokinumab were not included. The objectives of each study, patient characteristics, and mode of evaluation differ, complicating comparisons among them. However, similar efficacy can be seen in all of them, with a good safety profile for tralokinumab to treat moderate-to-severe AD.
ADCT, Atopic Dermatitis Control Tool; AE, adverse event; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; IGA, Investigator's Global Assessment; IQR, interquartile range; PP-NRS, Peak Pruritus Numerical Rating Scale; S-NRS, Sleep-Numerical Rating Scale; SCORAD, SCORing Atopic Dermatitis; SD, standard deviation.
Table references:
Pezzolo E, Naldi L. Tralokinumab in the treatment of resistant atopic dermatitis: An open-label, retrospective case series study. J Eur Acad Dermatol Venereol. 2023;37:e644-e645.
Moennig E, Traidl S. Real-world experience with tralokinumab in a patient with recalcitrant atopic dermatitis: A case report. Clin Cosmet Investig Dermatol. 2022;15:2825-2830.
Schlösser AR, Shareef M, Olydam J, et al. Tralokinumab treatment for patients with moderate-to-severe atopic dermatitis in daily practice. Clin Exp Dermatol. 2023;48:510-517.
De Greef A, Ghislain PD, Bulinckx A, et al. Real-life experience of tralokinumab for the treatment of adult patients with severe atopic dermatitis: A multicentric prospective study. Clin Drug Investig. 2023;43:299-306.
Gargiulo L, Ibba L, Vignoli CA, et al. Tralokinumab rapidly improves subjective symptoms and quality of life in patients with moderate-to-severe atopic dermatitis: A real-life 16-week experience. J Dermatolog Treat. 2023;34:2216815.
Pezzolo E, Schena D, Gambardella A, et al. Survival, efficacy and safety of tralokinumab after 32 and 52?weeks of treatment for moderate-to-severe atopic dermatitis in adults: A multicentre real-world study. J Eur Acad Dermatol Venereol. 2024;38:e11-e13.
Pezzolo E, Gambardella A, Guanti M, et al. Tralokinumab shows clinical improvement in patients with prurigo nodularis-like phenotype atopic dermatitis: A multicenter, prospective, open-label case series study. J Am Acad Dermatol. 2023;89:430-432.
García Castro R, Heras Mendaza F, Santiago Sánchez-Mateos DI, et al. First short-term effectiveness and security data of tralokinumab in severe atopic dermatitis in real clinical practice [published online ahead of print, 2023 May 2]. Dermatitis. 2023. doi: 10.1089/derm.2023.0030.
The added value of this study is the need to have data available on tralokinumab in the routine management of moderate-to-severe AD, being the second largest study ever conducted in Spain with the longest evaluated response period, assessing patients up to 24 weeks after starting treatment.10 The limitations of our study lie in its small sample size. Future studies should evaluate the response of tralokinumab in the routine clinical practice with larger populations and longer follow-ups.
Conflicts of interestNone declared.