Immune checkpoint inhibitors (ICIs) can cause immune-mediated cutaneous adverse events, including sarcoid-like reactions. The aim of this study was to retrospectively analyze clinical and histologic data from patients who developed cutaneous sarcoid-like reactions between 2019 and 2022 while under treatment with ICIs. We studied 7 patients (6 women and 1 man) with a median age of 65years. Median time to onset of symptoms was 4months. The most common presentation was papular sarcoidosis of the knees followed by subcutaneous sarcoidosis. Diagnosis was confirmed histologically in all cases, and no differences were observed relative to idiopathic sarcoidosis. Discontinuation of ICI therapy was required in just two patients. ICI-induced sarcoid-like reactions tend to be mild and generally do not require treatment discontinuation. Histologic confirmation is essential for distinguishing these reactions from tumor progression.
Los inhibidores de puntos de control inmunitario (ICI) pueden producir toxicidades cutáneas inmunomediadas entre las que se encuentran las reacciones sarcoideas. Nuestro objetivo fue analizar retrospectivamente los datos clínicos e histológicos de los pacientes en tratamiento con ICI que desarrollaron reacciones sarcoideas cutáneas entre 2019 y 2022. Se incluyeron siete pacientes (seis mujeres y un varón, con una edad mediana de 65años). La mediana de tiempo de instauración de la clínica fue de 4meses y la forma de presentación más frecuente fue la sarcoidosis papulosa de las rodillas, seguida de la sarcoidosis subcutánea. En todos se confirmó el diagnóstico histológicamente y no se observaron diferencias respecto a la sarcoidosis idiopática. Solo en dos casos fue preciso retirar la inmunoterapia. Las reacciones sarcoideas por ICI suelen ser leves y no suelen requerir la interrupción del tratamiento. Es fundamental obtener una confirmación histológica para distinguirlas de la progresión tumoral.
Immune checkpoint inhibitors (ICIs) are increasingly used in the treatment of various types of malignant neoplasm. Their mechanism of action favors the onset of a new range of toxicities, immune-related adverse events, which include sarcoid-like reactions.1
The objectives of the present study were to address the clinical and histologic characteristics of skin lesions compatible with sarcoid-like reactions to ICIs in our hospital and to identify differences with respect to idiopathic sarcoidosis lesions.
Material and MethodsWe retrospectively studied cases of sarcoid-like reactions to ICIs diagnosed between January 2019 and September 2022 and identified from the specific register of the Dermatology Department of Hospital Universitario de Bellvitge, Barcelona, Spain. Ours is a university hospital that provides tertiary care to a catchment population of approximately 1million people. We recorded demographic data (age, sex, underlying neoplasm, immunotherapy received, history of autoimmune disease), clinical data (type of skin lesion and area affected, extracutaneous involvement, severity, and need for specific treatment and/or suspension of ICIs), laboratory data (kidney function and angiotensin-converting enzyme values, blood calcium, and liver enzymes), and histologic data (type of granuloma, area affected by granulomas, and presence of foreign bodies, lymphocytic corona, necrosis, or fibrosis, as well as epidermal changes) and performed a descriptive study.
ResultsWe identified 7 patients (6 women, 1 man), with a mean age of 65years (range, 44-74). No patient had a history of autoimmune disease before starting immunotherapy.
Six patients received immunotherapy alone (anti-PD1 agent in 3 and anti-PDL1 agent in 3) and 1 received a combination of an anti-PDL1 agent and an anti-CTLA-4 agent. The most common underlying tumors were melanoma (3patients), followed by endometrial carcinoma (2), thyroid carcinoma (1), and lung carcinoma (1).
The median time from initiation of treatment to diagnosis of sarcoidosis was 4months (range, 2-9 months), and the most frequent cutaneous presentation was papular sarcoidosis of the knees, which affected 5 of the 7 patients. Three patients had subcutaneous nodules, 1 had infiltrated plaques on the forehead and upper back, and 1 had infiltration by granulomas from pre-existing xanthelasmas (previously published case).2
At the extracutaneous level, the most frequently affected organs were the hilar and mediastinal lymph nodes (4patients), the lung (3patients, in the form of multiple small pulmonary nodules), the joints,2 and the kidney (acute granulomatous tubulointerstitial nephritis). No ocular, cardiac, hepatic, or central nervous system involvement was recorded.
All patients underwent a skin biopsy for histological confirmation of the diagnosis, as well as biopsy of the other organs affected. Tumor progression was ruled out in all cases, as were fungal and mycobacterial infections (based on staining and tissue culture).
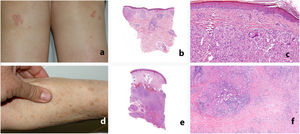
Study of the patients’ skin biopsies revealed granulomas formed by aggregates of epithelioid histiocytes with a sparse lymphocytic component. In the patients with papular sarcoidosis of the knees, the granulomas were located mainly in the superficial dermis, with frequent presence of foreign bodies (found in 3 of the 4patients who underwent biopsy). In contrast, in the patients with subcutaneous sarcoidosis, the granulomas were almost limited to the subcutaneous cellular tissue and surrounded by abundant lymphocytes; fibrosis was observed in 2 of the 3patients (Table 1 and Fig. 1). In terms of histology, no differences were observed with respect to idiopathic sarcoidosis.
Histology Findings for Skin Biopsy Specimens Taken From the 7 Patients Studied.
| Location of biopsy | Type of granuloma | Location of granulomas | Foreign body | Lymphocytic coronaa | Central necrosis | Epidermis | Interface dermatitis | Eosinophils | Fibrosis | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Forearm | Sarcoid | Subcutaneous | − | ++ | − | Normal | − | − | Yes |
| 2 | Knee | Sarcoid | Sup dermis | Yes | − | − | Normal | − | − | − |
| 3 | Back | Sarcoid | Sup dermis | − | − | − | Normal | − | − | − |
| 4 | Forearm | Sarcoid | Subcutaneous | − | ++ | Yes | Normal | − | − | Yes |
| Knee | Sarcoid | Sup + retic dermis | − | - | Yes | Normal | − | − | − | |
| 5 | Knee | Sarcoid | Sup dermis | Yes | + | − | Focal hyperkeratosis | − | − | − |
| 6 | Finger | Sarcoid | Subcutaneous | − | + | − | Normal | − | − | − |
| 7 | Eyelid | Sarcoid | Dermis | Yes | + | − | Normal | − | − | − |
| Knee | Sarcoid | Sup + retic dermis | Yes | + | − | Orthokeratosis | − | − | − |
Abbreviations: retic, reticular; sup, superficial.
Clinical and histologic appearance of sarcoid-like reactions to immune checkpoint inhibitors. A-C, Papular sarcoidosis of the knees. The granulomas are located mainly in the superficial dermis, with frequent presence of foreign bodies. D-F, Subcutaneous sarcoidosis. The granulomas are almost limited to the subcutaneous cellular tissue and are surrounded by abundant lymphocytes and fibrosis.
Laboratory tests revealed increased angiotensin-converting enzyme values in 3patients; no patients had hypercalcemia or increased liver enzymes. Lung function tests performed in patients with lung or lymph node involvement revealed reduced diffusing capacity of the lung for carbon monoxide.
Most patients had mild and self-limiting symptoms, with no need for specific treatment or suspension of ICIs, except in 2 cases, where the clinical course was more aggressive, with kidney and lung involvement requiring discontinuation of immunotherapy. The patients started systemic corticosteroids at 0.5 to 1mg/kg/d, followed by maintenance treatment with hydroxychloroquine.
As for the underlying tumor, 4patients achieved complete or partial remission, and 2patients died owing to tumor progression. The disease remained stable in 1 patient.
DiscussionSarcoidosis associated with immunotherapy based on ICIs affects around 5% of patients at 5months of treatment3,4 and is more frequent in patients receiving anti–CTLA-4 agents, especially those receiving double immunotherapy, in whom the disease may appear earlier and be more severe.4–7
Diagnosis is confirmed by histology, which makes it possible to differentiate the disease from tumor progression, especially in cases of pulmonary and mediastinal lesions, which may be indistinguishable in imaging tests, even in positron-emission tomography–computed tomography.8 The dermatologist plays a key role, since the skin is involved in 1 in every 5 patients and is fully accessible for biopsy.7 A confirmed diagnosis will dictate the approach to the cancer treatment prescribed.
Most sarcoid-like reactions to ICIs are mild and self-limiting, as we observed in the patients we studied. Therefore, the approach taken tends to be less aggressive, with treatment limited to patients with notable symptoms or cardiac, renal, neurologic, or ocular involvement.4,9 The treatment administered in these cases comprises courses of systemic corticosteroids and hydroxychloroquine; immunosuppressants are rarely necessary.10 Skin manifestations can be treated with topical corticosteroids, although they frequently resolve spontaneously.3,6
According to the literature, immunotherapy is suspended in more than half of cases on diagnosis of sarcoid-like reactions in order to rule out the trigger.7 However, there is increasing evidence that these reactions follow a benign course and that, in mild cases, maintaining immunotherapy does not necessarily imply worsening.2 Therefore, given the importance of treating the tumor for survival, it is thought that priority should be given to maintaining ICIs, except in severe cases.7,9,10
Recent studies suggest that prognosis for the underlying tumor is favorable in patients receiving ICIs who develop sarcoid-like reactions2,7,11–13 and that survival is greater than in control groups, even when adjusted for variables such as age, type of treatment, lines of treatment received, and underlying tumor.14
Our study is limited by its single-center, retrospective design and small sample.
In summary, cutaneous sarcoid-like reactions to immunotherapy clinically and histologically mimic the various types of cutaneous sarcoidosis. The clinical course is usually mild and self-limiting, and onset has been associated with greater survival; therefore, whenever possible, priority should be given to maintaining immunotherapy. Even though clinical, laboratory, and radiologic findings may be suggestive of sarcoid-like reaction, histologic confirmation should be sought, since the main differential diagnosis is with tumor progression.
FundingThe authors declare that no funding was received for this manuscript.
Conflicts of InterestThe authors declare that they have no conflicts of interest.