In recent years, hematopoietic stem cell transplantation (HSCT) has revolutionized the treatment of various hematological and non-hematological diseases. Its implementation is not stranger to risks and involves a significant rate of complications, including mucocutaneous adverse events. We present a narrative review of the mucocutaneous alterations observed after HSCT. Among these, acute and chronic graft-versus-host disease (GVHD) stand out, whose diagnosis and treatment can be challenging. Other common conditions include cutaneous adverse reactions and infections with mucocutaneous involvement. Additionally, various studies indicate that these individuals may have a higher rate of mucocutaneous neoplasms. Early identification and management of these complications, along with a multidisciplinary approach, are essential to improving these patients’ quality of life and long-term outcomes. Furthermore, it is advisable to screen for skin cancer in these individuals, especially if they have other associated risk factors.
En los últimos años, el trasplante de progenitores hematopoyéticos (TPH) ha revolucionado el tratamiento de diversas enfermedades hematológicas y no hematológicas. Su realización no está exenta de riesgos y conlleva una tasa significativa de complicaciones, entre ellas mucocutáneas. Presentamos una revisión narrativa de las alteraciones mucocutáneas observadas tras TPH. Entre ellas, destacan la enfermedad de injerto contra receptor (EICR) aguda y crónica, cuyo diagnóstico y tratamiento pueden ser notoriamente complejos. Otras patologías frecuentes son las toxicodermias y las infecciones con afectación mucocutánea. Además, diversos estudios muestran que estos individuos pueden tener una mayor tasa de neoplasias mucocutáneas. La identificación y manejo temprano de estas complicaciones, junto con un enfoque multidisciplinar, son esenciales para mejorar la calidad de vida y los resultados a largo plazo de estos pacientes. Asimismo, es recomendable el cribado de cáncer cutáneo en estos individuos, especialmente si presentan otros factores de riesgo.
Hematopoietic stem cell transplantation (HSCT), which includes bone marrow, peripheral blood, and umbilical cord blood transplants, involves administering healthy hematopoietic stem cells to patients with dysfunctional bone marrow due to malignant hematological diseases, bone marrow failure syndromes, or severe immunodeficiencies. HSCT can be autologous or allogeneic, depending on whether the hematopoietic cells come from the patient or a donor, respectively. Currently, HSCT is established as the treatment of choice for various severe malignant and non-malignant hematological conditions.1 According to data from the Spanish National Transplant Organization (ONT), more than 3500 HSCTs were performed in 2022—double the number conducted in 2002.2
HSCT includes a conditioning phase, in which chemotherapy and/or radiotherapy is administered to prepare the recipient's bone marrow and eliminate neoplastic cells (Fig. 1). One or two days later, the infusion phase is conducted. The patient then enters the aplasia period, during which immunity is significantly reduced due to lack of blood cell production by the bone marrow. This is a critical stage, during which the patient may experience severe anemia, bleeding, and infections. Finally, the engraftment phase occurs, beginning when the transplanted stem cells start producing new cells in the marrow. The time to engraftment varies depending on the type of transplant and patient-specific conditions, ranging from 11 to 40 days.3
After HSCT, patients may experience numerous mucocutaneous complications resulting from the transplant itself, the immunosuppressive therapy, or the graft-versus-host effect. A recent study reported that up to 45% of HSCT recipients developed some type of skin eruption within year 1 after receiving the transplant, with rates rising to 60–70% in the long term. These complications can significantly impact patients’ quality of life and may even be life-threatening.4 This article reviews the mucocutaneous changes observed in HSCT recipients, focusing on acute and chronic graft-versus-host disease (GVHD), drug eruptions, infections, and mucocutaneous neoplasms.
Graft-versus-host disease after hematopoietic stem cell transplantationIn GVHD, the immune cells from the graft (transplant) recognize the recipient (patient) as foreign and attack their tissues. GVHD is categorized into acute (aGVHD) and chronic (cGVHD) forms. Classically, they were differentiated by timing (before or after day 100 post-HSCT), but current classification is based on different pathophysiologic mechanisms and clinical presentations (Table 1).5–7 GVHD can affect any organ, although skin and mucous membranes are most widely involved (20–70%), and this involvement often aids diagnosis. It is an intrinsic complication of allogeneic HSCT (allo-HSCT), where donor cells differ from the recipient's, which significantly contributes to morbidity and mortality, being the most common cause of death after hematologic malignancy relapse.6
GVHD classification.
| Type | Time since HSCT | Acute GVHD signs/symptomsa | Chronic GVHD signs/symptomsb | |
|---|---|---|---|---|
| Acute GVHD | Classic | ≤100 days | + | − |
| Persistent, recurrent, or late-onset | >100 days | + | − | |
| Chronic GVHD | Classic lichenoid | No time limit, typically earlier | − | + |
| Classic sclerodermiform | No time limit, typically later | − | + | |
| Other patterns of chronic GVHD | Variable | − | + | |
| Overlap syndrome | − | − | + | + |
GVHD: graft-versus-host disease; HSCT: hematopoietic stem cell transplantation.
Chronic GVHD signs/symptoms: skin: sclerodermiform, lichenoid, or other patterns; mouth: dry syndrome, lichenoid, erosive, etc.; genital: dry syndrome, lichenoid, erosive, etc.; gastrointestinal: chronic diarrhea, abdominal pain, hepatic dysfunction; pulmonary: bronchiolitis obliterans; muscular, joint, neurological: peripheral or central neuropathy, etc.
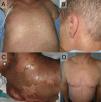
aGVHD frequently first affects the skin and mucous membranes. The liver and intestines are typically affected next. The classic aGVHD triad includes skin rash, hyperbilirubinemia, and diarrhea. Traditionally, it appears and resolves within the first 100 days post-HSCT (often developing between days 30–40). However, it may also occur later (late onset), persist beyond 100 days, or recur after resolution.7 It is graded into 4 stages: grade 1 (+): <25% of total body surface area (TBSA) involved; grade 2 (++): 25–50% TBSA; grade 3 (+++): 50–75% TBSA; grade 4 (++++): >75% TBSA.7 Cutaneous presentation (Fig. 2) typically begins with dysesthesias, pruritus, erythema, or edema, progressing into a morbilliform rash, often folliculotropic and trunk-predominant, then becoming confluent and spreading centrifugally. Palmar, plantar, and retroauricular involvement is a typical finding as well. Severe cases may develop epidermal detachment and blistering. The oral, genital, nasal, and ocular mucosa may also be involved, presenting as mucositis.8
Acute cutaneous GVHD. A. Maculopapular rash affecting the trunk, with follicular predominance. B. Retroauricular involvement, characteristic of the disease. C. Epidermal detachment in a patient with grade 4 aGVHD. D. Maculopapular rash affecting the trunk, with follicular predominance. This patient was ultimately diagnosed with a piperacillin–tazobactam-induced drug eruption. Note the diagnostic challenge with GVHD, as this case is very similar to patient A.
Initial suspicion of aGVHD is based on clinical findings: the triad of rash, diarrhea, and hyperbilirubinemia—although not all signs may be present or other organs may be affected. Skin biopsy is not pathognomonic. Histologically, aGVHD can be categorized into grade I: focal vacuolar changes at the basal membrane, with sparse lymphocytic infiltrate; grade II: keratinocyte necrosis with more evident vacuolar damage; grade III: keratinocyte apoptosis, dermoepidermal junction obliteration, lichenoid dermal infiltrate; grade IV: total epidermal necrosis with dermoepidermal separation.9
Differential diagnosis is complex (Table 2, Fig. 2). Rashes due to drug eruptions or viral infections may mimic aGVHD. In this context, concurrent diarrhea and hyperbilirubinemia support the diagnosis of aGVHD; a new drug exposure supports drug eruption; and respiratory symptoms or PCR/serologic positivity support infection.5,8,10 Histologically, sparse eosinophils and absence of spongiosis in aGVHD may help distinguish it from drug eruptions.11,12 Similarly, immunohistochemical markers such as elafin13 and more recently, microRNA expression,14 have been proposed for aGVHD diagnosis, but validation is ongoing and diagnosis may remain uncertain despite thorough work-up.
Differential diagnosis of mucocutaneous signs of acute GVHD.a
| Differential diagnosis | Key clinical clues | Additional tests |
|---|---|---|
| Drug eruptions | Triggering drug exposure; absence of other suggestive GVHD symptoms.bAtypical targets or epidermal detachment (SJS/TEN). Lymphadenopathy and facial edema (DRESS).Pustules and fever (AGEP).Retroauricular, folliculotropic trunk, or palmoplantar involvement favors GVHD. | Histology: absence of adnexal involvement, presence of spongiosis and dermal eosinophils favors drug eruptions, though not specific.Lab test results: elevated liver enzymes, renal or cardiac dysfunction suggest DRESS; neutrophilia suggests AGEP; cholestatic liver pattern suggests GVHD. |
| Viral exanthem | More common in children; associated cough, conjunctivitis, rhinorrhea, reactive lymphadenopathy; typically non-pruritic; absence of other GVHD signs. | Viral serologies, viral PCR. |
| Engraftment syndrome | Occurs within the first 2 weeks post-HSCT (including autologous transplants). Common features include fever, pulmonary edema, weight gain, absence of diarrhea. | Lab test results: absence of transaminitis supports engraftment syndrome. |
| Connective tissue autoimmune diseases (e.g., lupus, dermatomyositis, morphea, systemic scleroderma) | Signs/symptoms of lupus (mucocutaneous, joint, muscle, lung, neuropsychiatric, etc.), dermatomyositis (cutaneous, muscle), morphea (indurated plaques, usually with prior inflammatory violet halo, without HSCT history), systemic sclerosis (scleroderma, Raynaud, digital ulcers, telangiectasias, calcinosis, musculoskeletal, dysphagia, lung involvement). Absence of other GVHD signs. | Lab test results: autoantibodies may be present.Muscle enzyme elevation in dermatomyositis. |
| Contact dermatitis | History of exposure to irritant/allergen, prior sensitization, sharply demarcated lesions, pruritus, absence of other GVHD signs. | Patch testing in allergic contact dermatitis. |
| Psoriasis | Well-defined erythematous-squamous plaques, Auspitz sign, predominance on extensor surfaces, scalp involvement, joint manifestations, and absence of other signs of GVHD. | Characteristic histology in psoriasis. |
| Lichen planus | Violaceous, pruritic, polygonal papules, often on wrists and ankles; Wickham striae; absence of GVHD signs. | Characteristic histology in lichen planus. |
| Zinc deficiency | Acral, periorificial dermatitis and alopecia; history of poor diet, alcoholism, or GI disease; improves with zinc supplementation; absence of GVHD signs. | Lab tests: serum zinc and alkaline phosphatase levels. |
DRESS: Drug Reaction with Eosinophilia and Systemic Symptoms; GVHD: graft-versus-host disease; AGEP: Acute Generalized Exanthematous Pustulosis; SJS/TEN: Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis; HSCT: hematopoietic stem cell transplantation.
The treatment of aGVHD depends on the grade and location of the disease. For localized grade I cutaneous aGVHD, topical corticosteroids may be used. In grade II aGVHD, systemic treatment with corticosteroids such as prednisone (1–2mg/kg/day, although lower doses may be sufficient) is required. For corticosteroid-refractory cutaneous aGVHD, a therapeutic option is extracorporeal photopheresis, which achieves complete response rates>80%, improves survival, and reduces mortality—especially when initiated within the first 35 days. UVA-1 and UVB phototherapy can also be beneficial in localized skin signs. Another alternative in refractory cases is the use of antithymocyte globulin (ATG). Among pharmacologic treatments, tacrolimus, mycophenolate mofetil, sirolimus, and Janus kinase inhibitors (JAK inhibitors), particularly ruxolitinib,15,16 are notable. The latter has recently been approved for steroid-refractory aGVHD.17
Chronic graft-versus-host diseasecGVHD is a multisystem disease potentially affecting any organ, with skin and oral mucosa being the most commonly involved sites—up to 80% of cases. Other affected organs include liver, eyes (dry eye syndrome), gut, and lungs. Musculoskeletal and psychological involvement is also common due to the chronicity of the disease.5,10,18,19
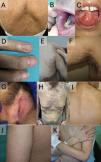
Mucocutaneous cGVHD is polymorphic, affecting skin, oral, and genital mucosa (Table 3, Fig. 3). In 2014, the NIH Consensus Project proposed an organ-specific classification of cGVHD.5,7
Clinical signs of mucocutaneous chronic GVHD.
| Skin | Hair | Nails | Oral mucosa | Genital mucosa |
|---|---|---|---|---|
| Lichen planus-likeLichen sclerosus-likeMorphea-likeFasciitis-likePoikilodermaPsoriasiformEczematous/dyshidroticSCLE-likePityriasis rosea-likeIchthyosiform/keratosis pilaris-likeHypopigmentationHyperpigmentationVitiligoAngiomatoid nodulesCalcinosis cutis | New-onset alopecia (especially patchy > diffuse).Can be scarring or non-scarringPremature gray hair discoloration | RoughnessThinning Breakage FragilityOnycholysisDorsal pterygiumAnonychia | Keratotic plaques, lichen planus-like lesionsMicrostomia due to sclerosis GingivitisMucositis, PseudomembranesUlcersXerostomiaMucosal atrophy Mucocele | Lichen planus-like lesions Vulvovaginal stenosis or scarringFissuresErosionsUlcersBalanitisPhimosis |
GVHD: graft-versus-host disease; SCLE: Subacute Cutaneous Lupus Erythematosus.
The “diagnostic” criteria from the NIH Consensus Project are highlighted in bold. These criteria refer to cutaneous signs that are sufficient for making a clinical diagnosis of chronic GVHD. The remaining criteria are referred to as “distinctive” for chronic GVHD, as they require the exclusion of other possible etiologies.
Chronic mucocutaneous GVHD. A–C. Lichen planus-like pattern affecting the back (A), labial and buccal mucosa (B), and tongue (C). D. Nail involvement with onycholysis and pterygium formation. E. Scleroderma-like pattern with secondary hyperpigmentation. F. Fasciitis-like pattern. G. Genital mucosa involvement with sclerodermiform, lichen sclerosus-like changes. H. Poikiloderma-like pattern. I. Lichen sclerosus-like pattern with extragenital involvement on the back. J. Keratosis pilaris-like pattern. K. Psoriasiform pattern.
Diagnosis is clinical and may be supported by skin biopsy. Histologically, 2 major patterns are recognized: lichenoid pattern: acanthosis, orthokeratotic and parakeratotic hyperkeratosis, band-like lymphocytic infiltrate, basal vacuolization, apoptotic keratinocytes, similar to lichen planus but with satellite cell necrosis. Sclerodermiform pattern: dermal sclerosis and periadnexal fat loss, resembling morphea or lichen sclerosus. Less common variants include fascial and psoriasiform patterns.9,20 These findings are nonspecific and must be interpreted clinically. Biomarkers for cGVHD are under investigation.21 Differential diagnoses include aGVHD, drug eruptions, viral infections, lichen planus, psoriasis, morphea, and systemic sclerosis.5,7
Regarding the treatment of mucocutaneous cGVHD, standardized therapeutic clinical practice guidelines are lacking, as most clinical trials exclude dermatologic outcomes. Proper skin care is essential, including general measures and emollients. First-line therapy for mild forms includes topical corticosteroids. Topical calcineurin inhibitors may be used as corticosteroid-sparing agents. In more severe cases, phototherapy (UVB or UVA1), extracorporeal photopheresis, rituximab, imatinib (especially in sclerodermiform forms), and more recently, ibrutinib and ruxolitinib10,15,16 are widely used. For oral and genital involvement, treatment is similar, with particular benefit noted from tacrolimus mouth rinses for oral lichenoid lesions.22 A multidisciplinary follow-up approach (hematology, dermatology, rheumatology, gynecology, among others) is essential given the chronicity of this disease and its potential complications.
Skin cancer after hematopoietic stem cell transplantationChronic immunosuppression is clearly associated with skin cancer in solid organ transplant (SOT) recipients.23–27 However, the relationship between HSCT and skin cancer is less well documented. HSCT recipients have an increased risk of secondary malignancies vs the general population.28–30 Solid tumors develop in up to 15% of patients 15 years after HSCT with myeloablative conditioning and account for 5–10% of late deaths.30 Regarding skin cancer (Table 4),29,32,50,52–61,65–67 published studies reveal an approximate incidence of 1–2% at the 5-year follow-up, 1–7% at the 10-year follow-up, and 6–10% at the 20-year follow-up.33 Several risk factors have been identified, including male sex, age at the time of HSCT, prior history of skin cancer, conditioning regimen, total body irradiation (TBI), use of voriconazole for antifungal prophylaxis, and presence of cGVHD.29–33 A recent systematic review and meta-analysis reported a standardized incidence ratio (SIR) for post-HSCT skin cancer of 7.21 (95%CI, 3.98–13.08), with an SIR of 2.25 (95%CI, 1.7–3.68) for autologous HSCT and 10.18 (95%CI, 5.07–20.43) for allogeneic HSCT. Risk factors for skin cancer included cGVHD—specifically for basal cell carcinoma and cutaneous squamous cell carcinoma (cSCC)—as well as male sex and voriconazole exposure for cSCC.34 GVHD, particularly cGVHD with mucocutaneous involvement, may be associated with increased skin cancer risk for several reasons: chronic inflammation of the skin and mucosa in cGVHD patients, and the greater need for immunosuppression in its treatment. Chronic inflammation has already been demonstrated to be an independent risk factor for skin cancer, particularly cSCC.49 Furthermore, voriconazole is a well-known phototoxic and carcinogenic drug, linked to the production of reactive oxygen species during its metabolism. Its use as antifungal prophylaxis in HSCT patients has been associated with skin cancer, particularly within the actinic keratosis–cSCC spectrum.50–52
Main studies evaluating the risk of actinic keratoses and skin cancer after HSCT.
| Source | Primary skin cancers (n) or patients with primary skin cancers (n) | Total no. of patients included | Age at HSCT (years), median (range) | Primary diseases | Time to diagnosis (years), median (range) | Cumulative incidence of each specific skin cancer | Identified risk factors |
|---|---|---|---|---|---|---|---|
| Cutaneous squamous cell carcinoma (cSCC) | |||||||
| Curtis et al.,53 2005 | 19 cSCC | 24,011 | 26.5 (3.5–61.3) (all cSCC cases) | ALL (6), AML (15), CML (14), lymphomas/MM (1), AA (17), FA (4), HGB (1) (all cSCC cases) | 7 (0.9–22.9) | 1.1% at 20 years | Combination of azathioprine+cyclosporine+steroids (all cSCC cases); azathioprine-containing therapies; long-term immunosuppression; chronic GVHD |
| Hasegawa et al.,54 2005 | 4 cSCC | 557 | 33.6 | CML (2), NHL (1), AA (1) | 4.37 | ND | ND |
| Leisenring et al.,55 2006 | 53 cSCC (includes mucosal) | 211 | 41.6 (6.8–71.4) (skin and mucosal SCC) | Hematological/marrow failure (10), malignant hematological disease (84), other malignant neoplasms (1) (skin and mucosal SCC) | 6.3 (0.3–24.8) | 3.5% at 20 years | Acute GVHD, chronic GVHD, younger age at transplant (<10 years) |
| Gallagher and Forrest55, 2007 | 4 cSCC | 926 | 49 | CML (1), AML (1), MDS (1), NHL (1) | 2.1 | ND | ND |
| Rizzo et al.,29 2009 | 19 cSCC | 28,874 | ND | ND | ND | ND | Chronic GVHD, male sex |
| Yokota et al.,32 2012 | 1 cSCC | 2062 | 46 | CML | 1.6 | ND | ND |
| Wojenski et al.,57 2015 | 27 cSCC | 381 | 55 (39–71) | Most common were AML (7), CLL (9), and MDS (6) | ND | 19% at 5 years | Male gender, underlying primary malignancy of CLL, transplant age, pre-HSCT skin cancer, extracorporeal photopheresis, UV therapy |
| Lupo-Stanghellini et al.,52 2016 | 6 cSCC | 302 | ND | ND | 3.5 (0.9–20) (both cSCC and BCC) | 3.2% at 3 years and 6.2% at 5 years (both cSCC and BCC) | Voriconazole |
| Omland et al.,58 2016 | 4 cSCC (2 allogeneic HSCT and 2 autologous HSCT) | 3302 | ND | ND | ND | ND | ND |
| Kuklinski et al.,50 2017 | 78 cSCC | 2638 | ND | ND | ND | ND | Chronic GVHD, male sex, voriconazole |
| Tanaka et al.,59 2017 | 4 (all oral squamous cell carcinomas) | 1060 | ND | ND | ND | 24.8% at 2 years (all oral cases) | ND |
| Wu et al.,60 2019 | 79 cSCC | 1974 | 58.1 | AML, ALL, CML, CLL, lymphomas, others | ND | IRR, 9.8; 95%CI, 7.7–12.3 | Age, CLL, chronic GVHD |
| Scott et al.,61 2020 | 62 cSCC | 872 | ND | AML, MPD, ALL, CLL, plasma cell disorders, CML, lymphomas, other non-malignant disorders | ND | 12.3% at 5 years; 95% CI, 8.5–16.3 | Chronic GVHD, Fitzpatrick skin type I |
| Cutaneous squamous cell carcinoma (cSCC) | |||||||
| Gruber et al.,62 2024 | 17 cSCC (includes 3 oral and 1 genital) | 266 | ND | AML | ND | 4.2% [95% CI (2.2, 7.2)] and 8.1% [95% CI (4.6, 12.8)] at 10 and 15 years, respectively | ND |
| Squamous cell carcinoma in situ (SCCis) | |||||||
| Gruber et al.,62 2024 | 8 (includes 1 genital) | 266 | ND | AML | ND | ND | ND |
| Basal cell carcinoma (BCC) | |||||||
| Hasegawa et al.,54 2005 | 5 BCC | 557 | 39.8 | ALL (2), CML (2), NHL (1) | 7.3 | ND | ND |
| Leisenring et al.,55 2006 | 201 BCC | 211 | 38.1 (2.9–71.3) | Hematological/marrow failure (7), hematological cancer (150), other malignant neoplasms (1) | 7.9 (0.5–30.2) | 6.5% at 20 years | TBI, fair skin color, chronic GVHD, younger age at transplant (< 10 years), leukemia/lymphomas/blood or malignant bone marrow disease as primary diagnosis |
| Basal cell carcinoma (BCC) | |||||||
| Gallagher and Forrest,56 2007 | 8 BCC | 926 | 41 | CML (3), ALL (1), AML (1), MDS (1), MM (1), NHL (1) | 7.6 | ND | ND |
| Borgmann et al.,63 2008 | 1 BCC | 490 | 7.8 | ALL | 20.3 | ND | ND |
| Schwartz et al.,64 2009 | 282 BCC | 6306 | ND | ND | ND | ND | TBI with higher risk for younger ages (< 10 years) at transplant and no excess risk for ages > 40 years at transplant, fair skin color for patients who had not undergone TBI, chronic GVHD in patients without TBI |
| Yokota et al.,32 2012 | 3 BCC | 2062 | 40 (17–50) | AML (2), ALL (1) | 8.4 (7.1–17.6) | ND | ND |
| Lupo-Stanghellini et al.,52 2016 | 19 BCC | 302 | ND | ND | 3.5 (0.9–20) (both cSCC and BCC) | 3.2% at 3 years and 6.2% at 5 years (both cSCC and BCC) | ND |
| Omland et al.,58 2016 | 24 BCC (7 allogeneic HSCT and 17 autologous HSCT) | 3302 | ND | ND | ND | ND | ND |
| Kuklinski et al.,50 2017 | 35 BCC | 2638 | ND | ND | ND | ND | Chronic GVHD, male sex, voriconazole |
| Wu et al.,60 2019 | 54 BCC | 1974 | 54.5 | AML, ALL, CML, CLL, lymphomas, others | ND | IRR, 2.5; 95%CI, 1.9–3.2 | CLL, reduced-intensity conditioning, acute GVHD, chronic GVHD |
| Basal cell carcinoma (BCC) | |||||||
| Scott et al.,61 2020 | 62 BCC | 872 | ND | AML, MPD, ALL, CLL, plasma cell disorders, CML, lymphomas, other non-malignant disorders | ND | 9.1% at 5 years; 95% CI, 5.4–12.8 | Age, phototherapy exposure prior to allogeneic HSCT |
| Melanoma | |||||||
| Baker et al.,65 2003 | 8 melanomas | 3372 | ND | ND | ND | O:E, 8:0.96, SIR, 8.3 (95% CI, 3.6–15.1), SIR, 6.7 | ND |
| Curtis et al.,53 2005 | 22 melanomas | 24,011 | ND | ND | ND | ND | ND |
| Brown et al.,66 2005 | 5 melanomas | 605 | ND | ND | North (O:3, 5:0.85) | ND | ND |
| Rizzo et al.,29 2009 | 18 melanomas | 28,874 | ND | ND | 1-to-4 year group (< 1 to > 10 years) | O:E ratio, 3.47, SIR, 1.5 | T-cell depletion, TBI, short latency period (< 1 year), female sex |
| Yokota et al.,32 2012 | 1 melanoma | 2062 | 51 | NHL | 2.1 | ND | ND |
| Mahindra et al.,67 2015 | 19 melanomas | 4161 | ND | ND | SIR 3.58 (CI, 1.82–6.29) | ND | ND |
| Omland et al.,58 2016 | 6 melanomas (2 allogeneic HSCT and 4 autologous HSCT) | 3302 | ND | ND | ND | ND | ND |
| Tanaka et al.,59 2017 | 1 melanoma | 1060 | ND | ND | ND | ND | ND |
| Wu et al.,60 2019 | 11 melanomas | 1974 | 48.6 | AML, ALL, CML, CLL, lymphomas, others | ND | IRR, 3.3; 95%CI, 1.7–5.9 | None |
Source: Adapted from the authors’ original work.
AA: aplastic anemia; FA: fanconi anemia; ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; BCC: basal cell carcinoma; CI: confidence interval; CLL: chronic lymphatic leukemia: CML: chronic myeloid leukemia; cSCC: cutaneous squamous cell carcinoma; GVHD: graft-versus-host disease; HGB: hemoglobinopathies; HSCT: hematopoietic stem cell transplantation; IRR: incidence rate ratio; MDS: myelodysplastic syndrome; MM: multiple myeloma; MPD: myeloproliferative disorder; ND: not described; NHL: non-hodgkin lymphomas; SIR: standardized incidence ratio; TBI: total body irradiation.
Due to the increased risk of skin cancer, several authors have recommended selective screening and ongoing dermatologic surveillance in these patients.34–36
Other mucocutaneous alterations after HSCT (Table 5)37,41–44,45,47,50,70Patients who undergo HSCT may present with numerous mucocutaneous alterations.44,45,47,50,70 In addition to the already mentioned GVHD, viral infections, drug eruptions, and secondary mucocutaneous neoplasms, attention should be paid to other infections and less frequent entities. The most common mucocutaneous infections after HSCT are those caused by the varicella-zoster virus, tunnel or catheter exit-site infections, and cutaneous signs of disseminated bacterial or fungal infections. Focal areas of bacterial cellulitis are common in the lower extremities, particularly in the context of edema due to heart failure, lymphedema, or impaired venous return.37,38 Molluscum contagiosum and cytomegalovirus infections are also relatively frequent. Of note, these infections may present atypically and more aggressively, given the immunosuppressed state and the polypharmacy in HSCT patients,39 and the possibility of post-transplant lymphoproliferative disorder associated with Epstein–Barr virus, though isolated skin lesions are rare.40
Main Studies Evaluating Mucocutaneous Alterations (Other than GVHD) After HSCT.
| Author/year | Sample size | Study type and conditions evaluated | Results |
|---|---|---|---|
| Canninga-van Dijk et al.37, 2003 | ND | Narrative review - All types of indications | Patients undergoing HSCT, in addition to viral rashes, are at higher risk of bacterial infections (especially Staphylococcus aureus pyoderma), herpes simplex and varicella-zoster virus infections, cytomegalovirus reactivation, molluscum contagiosum, or fungal infections.- Their presentation can be atypical and more aggressive given the context.- Of note, the possibility of EBV-associated post-transplant lymphoma, although presentation with isolated cutaneous lesions is rare. |
| Mabuchi et al.41, 2012 | 1 | Isolated case report- Single-center- NHL | Psoriasis can develop de novo after HSCT from a non-psoriatic donor.a In this case, 10 years after allogeneic HSCT in a patient with NHL. |
| Khalil et al.68, 2014 | 92 | - Retrospective cohort study- Single-center- Non-malignant diseases | Six patients (6.5%) were diagnosed with vitiligo unrelated to chronic GVHD, 6 (6.5%) with autoimmune hemolytic anemia, 6 (6.5%) with idiopathic thrombocytopenia, 3 (3.3%) with mild leukopenia, 2 (2.2%) with aplastic anemia, and one (1.1%) with autoimmune thyroid disease.- Autoimmune complications were more frequent in patients who underwent HSCT for metabolic disorders. |
| Kato et al.47, 2015 | 1 | Isolated case report- Single-center- T-cell Lymphoblastic leukemia | - HSCT could be a risk factor for bullous pemphigoid in patients undergoing HSCT. Therefore, in cases of suspicious clinical presentation, it should be ruled out with clinical examination, biopsy, and serology for anti-epidermal basement membrane antibodies. |
| Bae et al.42, 2016 | 2457 HSCT recipients vs 8241 controls | - Retrospective cohort study- Multi-center- All diagnoses | - An association with vitiligo was found in HSCT patients, independently of the presence of GVHD and higher than in the control group.- The risk factors most associated with the development of vitiligo were allogeneic HSCT and bone marrow as the source. |
| Song et al.44, 2017 | 85 HSCT recipients vs 85 controls | Prospective cohort study- Single-center- All diagnoses | - Children who underwent HSCT had significantly more nevi than controls (median [range]: 44 (0–150) vs. 11 (0–94), p=0.0001).- Children with HSCT had significantly more nevi > 5mm in diameter and more atypical nevi than controls.- Factors associated with a higher number of nevi included malignant indication for HSCT, pre-transplant chemo, TBI exposure, and myeloablative conditioning. |
| Bresters et al.43, 2017 | 263 | Retrospective cohort study- Single-center- All diagnoses | - The percentage of permanent alopecia was 15.6% (41/263 patients).- A conditioning regimen with busulfan and busulfan plus fludarabine (OR, 5.7 [CI, 2.5–12.7] and OR; 7.4 [CI, 3.3–16.2], respectively) was the main risk factor and was associated with alopecia regardless on the presence of acute/chronic GVHD. |
| Huang et al.48, 2018 | 85 | Retrospective cohort study- Single-center- All diagnoses | - 14% (n=12) of patients developed vitiligo; 16% (n=14) developed psoriasis/sebopsoriasis; 25% (n=21) developed alopecia; and 6% (n=5) developed nail alterations.- Factors significantly associated with vitiligo, independent of GVHD, included an indication of primary immunodeficiency and younger age at transplant (<10 years).- Factors significantly associated with alopecia, independent of GVHD, were busulfan conditioning and a family history of early-onset androgenic alopecia.- The only risk factor identified for nail alterations was a history of chronic GVHD. |
| Miyagi et al.45, 2023 | 2 | - Two case reports and literature review- Single-center | - Dermatomyositis can be a late complication of HSCT, and it's essential to rule it out in patients undergoing HSCT who present with compatible cutaneous and muscular symptoms. |
GVHD: graft-versus-host disease; CI: confidence interval; NHL: non-hodgkin lymphoma; ND: not described; OR: odds ratio; Chemo: chemotherapy; TBI: total body irradiation; HSCT: hematopoietic stem cell transplantation.
Other dermatoses include de novo development of psoriasis,41 vitiligo in patients without other signs of chronic GVHD,42 alopecia in patients without other signs of cGVHD,43 or the appearance of melanocytic nevi in children.44 Recently, several cases of dermatomyositis have been reported in patients previously subjected to HSCT, some of them with severe pulmonary involvement,45,46 and 1 case of post-HSCT bullous pemphigoid.47 A recent study described late cutaneous alterations in children who had undergone HSCT, highlighting a high incidence of vitiligo, psoriasis/sebopsoriasis, alopecia, and nail changes—particularly in children with cGVHD, age<10 years at the time of HSCT, and with primary immunodeficiency as the underlying condition at transplant.48
DiscussionThis review presents the main mucocutaneous changes in HSCT recipients. GVHD is the most prominent complication due to its frequency and severity. The acute form poses a complex differential diagnosis—especially vs drug eruptions and viral infections. Accurate diagnosis depends on a thorough clinical history.5,8,10 Treatment includes topical/systemic corticosteroids and, more recently, ruxolitinib, approved as second-line therapy.15–17 Chronic GVHD is strikingly polymorphic, with more than 10 possible skin signs, including lichenoid and sclerodermiform types, and can also affect oral, genital, hair, or nail sites.5,7 This highlights the need for dermatologic evaluation and multidisciplinary care.
ConclusionsHSCT has revolutionized the treatment of various hematologic and non-hematologic diseases but carries a significant risk of mucocutaneous complications. Among them, GVHD—acute and chronic—remains a major diagnostic and therapeutic challenge. Other frequent issues include skin infections and drug eruptions. Early identification and a multidisciplinary approach are essential for improving quality of life and long-term outcomes. Skin cancer screening and photoprotection advice are also recommended, especially in at-risk individuals.
Conflicts of interestNone declared.