Over the last few years, the use of low-dose oral minoxidil to treat androgenetic alopecia has brought about a small revolution in the field of dermatology in general, and trichology in particular.1 Oral administration appears to provide important benefits in terms of convenience, cosmesis, cost-efficiency, co-therapy, and compliance.2 However, although considered safe the use of this regimen requires an understanding of the drug and its adverse effects. The most frequently described adverse effects are hypertrichosis, postural hypotension, tachycardia, and pretibial edema.3 Below, we describe 2 cases of morning periorbital edema associated with low doses of oral minoxidil, an effect rarely reported in the literature.4
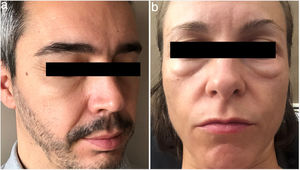
The first patient was a 40-year-old man with androgenetic alopecia (Hamilton–Norwood stage II) and no previous history of disease. He had received no previous treatment for his alopecia. The patient began treatment with oral minoxidil in monotherapy (5mg/d), taken nightly. After 4 weeks of treatment, the patient noticed swelling of the lower eyelids in the morning (Fig. 1A). This swelling resolved spontaneously during the day, and had no impact on the patient's daily life, nor was it accompanied by edema in other locations or other clinical signs such as urticaria or respiratory distress. The patient was diagnosed with periorbital edema associated with oral minoxidil treatment. With the patient's consent, the dose of minoxidil was reduced to 3mg/day, resulting in complete resolution of the symptoms within a few days.
The second patient was a 37-year-old woman with no previous history of disease who began treatment with oral minoxidil (1mg/d, taken nightly) for androgenetic alopecia (Ludwig stage Ib). After 2 weeks of treatment, the patient reported the appearance of morning periorbital edema (Fig. 1B) that resolved spontaneously within 60minutes. The edema was not accompanied by any additional clinical signs. It was decided to withdraw treatment for 12 weeks, resulting in complete resolution after 7 days. Treatment was subsequently reinitiated at a dose of 0.5mg/day, taken nightly, without the reappearance of edema.
Edema associated with the use of oral minoxidil is widely documented in the literature.1–6 This effect is directly due to the drug's induction of capillary vasodilatation. The resulting edema most often affects the lower limbs, and develops in 1–10% of cases, depending on the dose used.3,5 Although it can occur in patients receiving doses as low as 1mg/day, the risk is much higher in those treated with 5mg/day.3 The likelihood of edema may be higher in obese patients and those receiving concomitant treatment with calcium channel blockers.5 It is not a serious adverse effect and does not result in long-term complications. It can even remit spontaneously after 2–3 months of treatment without the need to alter the dose regimen.5 In the most severe cases it can markedly impact quality of life, and may necessitate discontinuation of treatment.6 While edema in the lower limbs is a relatively common adverse effect, its appearance in other locations, such as the face, is unusual, accounting for around 0.3% of cases.4
While periorbital edema associated with low doses of oral minoxidil is very rare, we believe it is very important to be aware of this adverse effect. Its appearance in the morning is probably a result of prolonged decubitus, hence its spontaneous resolution during the day. In most of the cases described, and in the cases reported here, this effect is mild, and although dose reduction may be required, treatment discontinuation is not usually necessary. However, the location is very striking, and can give rise to confusion with allergic angioedema, which requires immediate attention. It is therefore important that patients are aware of this possible adverse effect and the accompanying warning signs.