Systemic treatment with immunotherapy or targeted therapy can significantly improve survival in patients with advanced (metastatic or high-risk) melanoma. Fifty percent of patients with melanoma have a BRAF mutation. Decisions on optimal sequencing of systemic treatments should take into account drug- and tumor-related factors and patient characteristics. Although the combination of ipilimumab and nivolumab is associated with the best survival outcomes, it is associated with significant toxicity. Targeted therapy may be a more favorable option in certain clinical situations. We review the literature on immunotherapy and targeted therapy in melanoma and present an algorithm for guiding decision-making on their use as first-line systemic treatments for advanced BRAF-mutated melanoma.
El paciente con melanoma avanzado, metastásico o de alto riesgo, cuenta con opciones de tratamiento sistémico, inmunoterapia y terapias dirigidas, que han mejorado significativamente su supervivencia. El 50% de los pacientes con melanoma presentan mutación del gen BRAF. La toma de decisiones en cuanto a la secuencia óptima de tratamiento sistémico debe tener en cuenta factores relacionados con el medicamento, factores clínicos del paciente, así como los propios del tumor. Aunque la combinación ipilimumab-nivolumab es la que proporciona mejores resultados de supervivencia en todos los pacientes, la toxicidad asociada y el perfil de las terapias diana las puede hacer recomendables como primera línea en pacientes en determinadas situaciones clínicas. El objetivo de esta revisión es proporcionar un algoritmo de toma de decisiones en cuanto a la primera línea de tratamiento sistémico, inmunoterapia vs. terapias dirigidas, en el paciente con melanoma avanzado con mutación BRAF.
Systemic treatments for metastatic melanoma have changed considerably in the past decade. The estimated 5-year survival rate for stage IV melanoma according to the seventh edition of the American Joint Committee on Cancer Staging Manual was less than 20%, but with the use of immunotherapy and targeted therapies (BRAF and MEK inhibitors), this has now increased to around 50%.1,2 Patients with BRAF-mutant melanoma can be treated with immunotherapy and/or targeted therapy, whereas immunotherapy is the only option for patients without BRAF mutations.
The aim of this review was to synthesize recent evidence-based research to guide decision-making on the use of first-line systemic treatments and optimal sequencing in patients with advanced BRAF V600-mutant melanoma.
Treatment Strategies for Advanced MelanomaTwo strategies are currently available for the systemic treatment of melanoma: immunotherapy and targeted therapy (Table 1). Both can be used in the adjuvant setting and the treatment of unresectable metastatic melanoma.
Drugs Currently Available for the Systemic Treatment of Melanoma in Clinical Practice.
| Treatment strategy | Drug | Mechanism of action | Route | Clinical practice dosage |
|---|---|---|---|---|
| Immune checkpoint inhibitors | Ipilimumab | CTLA-4 blockade | IV | Ipilimumab 3mg/kg every 3wk up to 4 doses |
| Nivolumab | PD-1 blockade | IV | Nivolumab 240mg every 2wk or 480mg every 4wk | |
| Pembrolizumab | PD-1 blockade | IV | Pembrolizumab 200mg every 3wk or 400mg every 6wk | |
| Ipilimumab plus nivolumab | CTLA-4 plusPD-1 blockade | IV | Nivolumab 1mg/kg and ipilimumab 3mg/kg every 3wk for the first 4doses. Nivolumab 240mg every 2wk or 480mg every 4wk | |
| Targeted therapy | Dabrafenib plus trametinib | BRAF and MEK inhibition | Oral | Dabrafenib 150mg/12hTrametinib 2mg/24h |
| Vemurafenib plus cobimetinib | BRAF and MEK inhibition | Oral | Vemurafenib 960mg/12hCobimetinib 60mg/24h for 21d, followed by 7days of rest, in continuous repeated cycles | |
| Encorafenib plus binimetinib | BRAF-MEK inhibition | Oral | Encorafenib 450mg/24hBinimetinib 45mg/12h |
Cutaneous melanoma is a highly immunogenic tumor, derived from a high mutational burden due to cumulative sun exposure.3 This immunogenicity results in the release of tumor autoantigens, which are processed by antigen-presenting cells and interact with lymphocyte receptors to trigger an immune response. These receptors, CTLA-4 and PD-1, are known as immune checkpoints. In melanoma, CTLA-4 and anti-PD-1 checkpoint immunotherapy blocks these antagonistic receptors and favors the antitumoral action of T cells.4
The goal of targeted therapy is to counter the effects of somatic mutations in tumor cells that promote uncontrolled cell proliferation.5BRAF mutations are present in approximately 50% of cutaneous melanomas; the most common mutation is the BRAF V600E mutation, found in 90% of cases.6,7 Targeted therapy thus can only be used to treat half of all patients with melanoma, and it is currently only effective when BRAF and MEK inhibitors are administered simultaneously.
Clinical and Treatment Factors Guiding Decisions on First-line Systemic TreatmentsWhen choosing a first-line systemic treatment, it is important to consider the differences between immunotherapy and targeted therapy and additional factors such as drug-related factors, individual patient characteristics (e.g., comorbidities), and tumor-related factors (e.g., behavior, progression) (Table 2).
Factors To Consider When Deciding on First-line Systemic Treatment for Patients With Advanced Melanoma.
| Drug-related factors | Tumor-related factors | Patient-related factors |
|---|---|---|
| • Survival results• Time to response• Duration of response• Toxicity• Administration route• Regulatory issues | • Tumor burden• Progression rate• Location of metastases: brain• Manifestations | • Autoimmune disease• Immunosuppressive agents: corticosteroids, other• Solid organ tumors• Performance status• Personal preference |
Long-term follow-up data from clinical trials of anti-PD1 monotherapy with nivolumab or pembrolizumab and combination therapy with BRAF and MEK inhibitors show 5-year overall survival rates of approximately 50%.2 Ipilimumab plus nivolumab, however, offers the best long-term survival outcomes, making it the general first choice for unresectable metastatic melanoma.8–10 It is, however, also associated with an increased risk of severe toxicity. Accordingly, first-line anti-PD1 monotherapy with nivolumab or pembrolizumab is also an acceptable choice.10,11
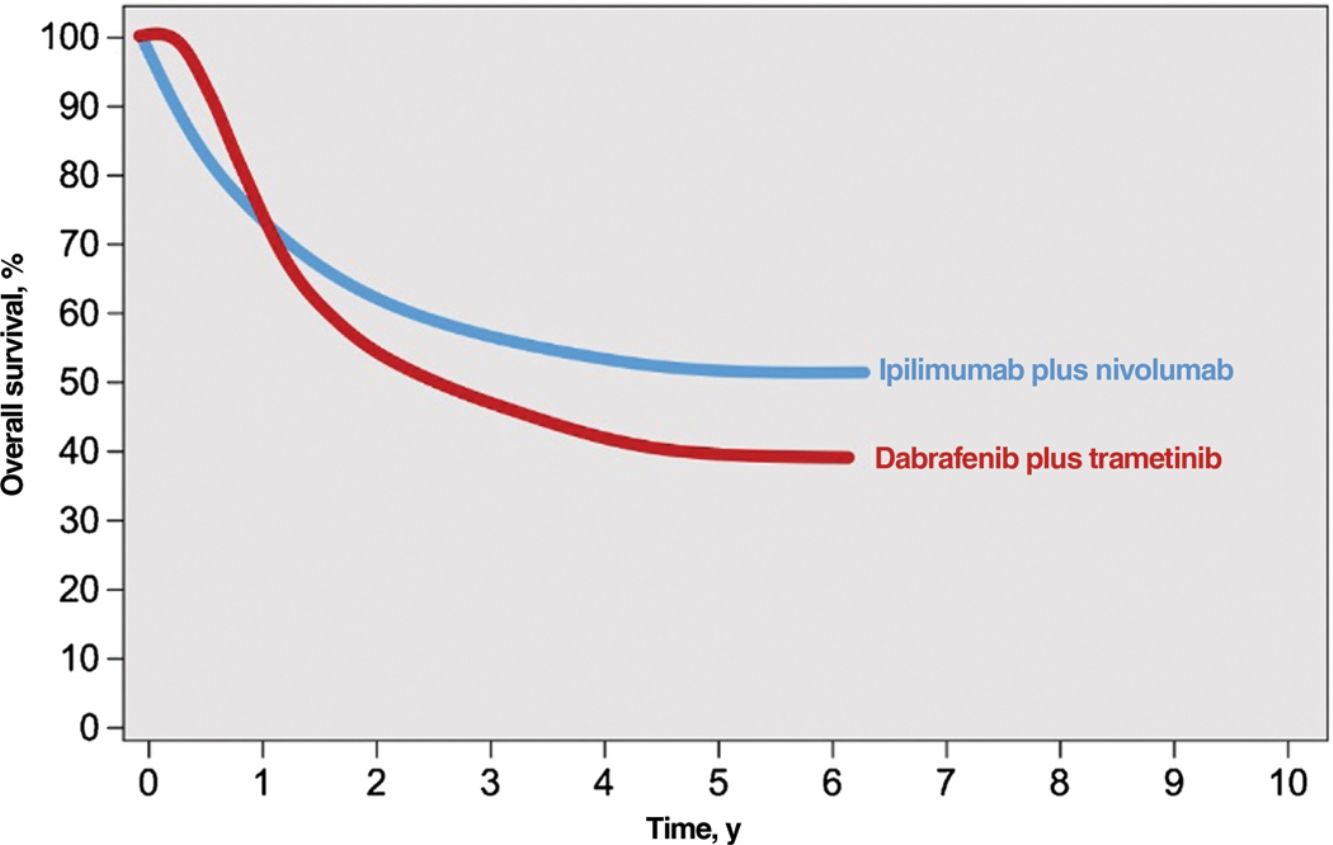
Immune checkpoint inhibitors (ICIs) and targeted agents are associated with considerably different treatment response patterns. Because of their mechanism of action, BRAF and MEK inhibitors produce faster responses than anti-PD1 or anti-CTLA-4 antibodies. The differences are evident in survival curves from clinical trials, which show a stronger initial plateau and better short-term survival in patients on targeted therapy and a sharper initial drop in survival, followed by clear survival benefits, in those on immunotherapy2 (Fig. 1). ICIs elicit more sustained responses, even after they are discontinued, whereas discontinuation of targeted agents is usually followed by rapid disease progression; BRAF and MEK inhibitors are therefore generally considered the treatment of choice for maintenance regimens.12,13 ICIs and BRAF and MEK inhibitors also have different safety profiles. ICIs often cause grade 3 or 4 adverse events, with incidence rates of 14% observed for anti-PD1 antibodies and 45% for the anti-CTLA-4 agent ipilimumab; in addition, these immune-mediated effects are often irreversible.14,15 BRAF and MEK inhibitors, by contrast, cause moderate to severe adverse events (grade 3-4 in 41% of patients) that are frequently reversible with dose adjustments or treatment discontinuation.16
Responses to combined anti-CTLA-4 immunotherapy and targeted therapy with BRAF and MEK inhibitors.
Source: Adapted from Michielin et al.2
Finally, ICIs and BRAF and MET inhibitors have different administration routes, an important consideration in the context of patient preferences (Table 1).
Individual Patient CharacteristicsIndividual patient-related factors, such as comorbidities or concomitant treatments, must be considered when evaluating first-line treatments for advanced melanoma. Presence of an underlying autoimmune disease has been associated with a significantly increased risk of immune-mediated adverse events in patients with melanoma receiving immunotherapy.17 Similar findings have been observed for anti-PD-1 agents in the treatment of advanced cancer, including melanoma. In particular, the authors observed a higher frequency of immune-related adverse events in patients with a previous autoimmune disease (65.9% vs. 39.9%), although the severity of effects (grade3-4) was similar to that observed in patients without a history of autoimmune disease.18 In another study, ipilimumab plus nivolumab triggered a flare in 33% of patient with a pre-existing autoimmune disease; the flares were particularly common in patients with inflammatory bowel disease or a rheumatologic disorder and in those on immunosuppressive therapy.19
An increased risk of disease progression and death has been observed in patients with cancer being treated with an ICI and corticosteroids for any reason (palliative treatment, brain metastases, etc.) (HR, 1.54; 95% CI, 1.24-1.91; P=.0001). A similar risk was observed for patients with melanoma (HR, 1.75; 95% CI, 1.07-2.88; P=.03).20 Another study reported a 50% organ rejection rate in kidney transplant recipients under treatment with ICIs, although a reduced immunosuppressive regimen was used in most patients.21 When immunosuppression was maintained, however, there was a striking reduction in organ rejection (6% retrievable allograft rejection with tumor response and 6% irretrievable allograft rejection with tumor response). The findings suggest that maintaining immunosuppression during ICI therapy might not diminish the effectiveness of immunotherapy and might even reduce the risk of allograft rejection.21 Nonetheless, and considering the available data, first-line targeted therapy could be a prudent choice for patients with BRAF-mutant melanoma who have an underlying autoimmune disease and/or are receiving immunosuppressive treatment, providing there are no other clinical factors that take precedence.
Performance status (PS) has been studied as a predictor of treatment response in melanoma, but clinical trials do not include patients with reduced functional status (a score of ≥2 on the Eastern Cooperative Oncology Group Performance Status [ECOG PS) Scale). One meta-analysis showed that ICIs improved survival regardless of ECOG PS.22 Studies of combined treatment with dabrafenib and trametinib, however, have found significant differences in progression-free survival in patients with reduced functional status (PS≥1 vs. PS=0).23,24
Tumor-Related FactorsTumor burden or volume and speed of progression and related symptoms must also be taken into account when evaluating first-line systemic treatments for patients with advanced melanoma.
A pooled analysis of data from clinical trials evaluating combination therapy with dabrafenib and trametinib showed that normal lactate dehydrogenase (LDH) levels, fewer than 3 metastatic sites, and less measurable disease were associated with better survival at 2 years.24 ICIs, however, have also been associated with survival benefits in patients with low tumor burden.25,26 The toxicity profile of BRAF and MEK inhibitors and the option of switching to immunotherapy in the event of progression are factors in favor of using first-line targeted therapy in patients with low tumor burden. A recent editorial on the role of BRAF and MEK inhibitors in melanoma defended the use of these agents in patients with low tumor burden, including candidates for adjuvant therapy. In this scenario, the results are similar to those observed with immunotherapy and, in addition, anti-PD-1 therapy is highly effective in patients who experience recurrence during BRAF-targeted therapy.27
Fast-acting BRAF and MEK inhibitors, however, could also be prioritized in the event of rapid, life-threatening symptomatic growth. Although response is short lasting, the priority is to bring the disease under control as quickly as possible. The European Association of Dermato-Oncology clinical guideline for melanoma recommends frontline BRAF and MEK inhibitors for patients with mutant tumors, high tumor burden, an aggressive course, or high LDH levels.11
The impact of tumor progression and associated symptoms is also influenced by the site of metastatic disease. A patient with progressive brain metastases, for example, will usually be administered symptomatic treatment with corticosteroids, most likely improving candidacy for first-line BRAF and MEK therapy.28 Combination immunotherapy with ipilimumab and nivolumab would be the first choice in patients with highly disseminated melanoma, even in non–life-threatening situations, as it has proven survival benefits.8,9,29,30
Proposals for Guiding Decisions on Systemic Treatments for Patients With BRAF-Mutant MelanomaAdjuvant and Neoadjuvant TherapyDisease-free survival benefits have been demonstrated for adjuvant immunotherapy and targeted therapy following complete resection of metastasis. Clinical practice guidelines thus recommend first-line adjuvant therapy with anti-PD1 antibodies for all patients with stage IIIA-IIID melanoma, regardless of BRAF status. For patients with BRAF V600-mutant melanoma, they recommend BRAF and MEK inhibitors10,11 (Table 3).
Indications and Regulatory Situation for Currently Available Immunotherapy and Targeted Therapy Drugs.
| Drug | Adjuvant treatment | Unresectable metastasis |
|---|---|---|
| Ipilimumab | Not authorized | AEMPS: treatment of advanced melanoma (unresectable or metastatic) in adults and adolescents aged ≥ 12 years |
| Nivolumab | AEMPS: adults with melanoma and lymph node metastasis after complete resectionIndication limited to patients with stageIIIC orIIID melanoma after complete resectionaFDA: patients with stageIIB-IIC melanoma after complete resection | AEMPS: treatment of advanced (unresectable or metastatic) melanoma in adults |
| Pembrolizumab | AEMPS: melanoma and lymph node metastasis after complete resectionIndication limited to patients with stageIIIC orIIID melanoma after complete resectionaFDA, EMA: adults and adolescents aged ≥12years with stageIIB, IIC, or III melanoma after complete resection | AEMPS: treatment in adults and adolescents aged ≥12years with advanced (unresectable or metastatic) melanoma |
| Ipilimumab plus nivolumab | FDA, EMA, AEMPS: not authorized | AEMPS: treatment of advanced (unresectable or metastatic) melanoma in adults |
| Dabrafenib plus trametinib | FDA, EMA: stage III melanoma with BRAF V600 mutation after complete resectionNot funded in Spainb | AEMPS: adults with unresectable or metastatic melanoma with a BRAF V600 mutation |
| Vemurafenib plus cobimetinib | FDA, EMA, AEMPS: not authorized | AEMPS: adults with unresectable or metastatic melanoma with a BRAF V600 mutation |
| Encorafenib plus binimetinib | FDA, EMA, AEMPS: not authorized | AEMPS: adults with unresectable or metastatic melanoma with a BRAF V600 mutation |
Abbreviations: AEMPS, Spanish Agency for Medicines and Medical Devices; EMA, European Medicines Agency; FDA, US Food and Drug Administration
The General Directorate of the Common Portfolio of Services of the Spanish National Health Care System has ruled that the use of pembrolizumab and nivolumab is limited to the adjuvant treatment of patients with stageIIIC orIIID melanoma with lymph node involvement who have undergone complete resection.
Based on the results of phaseIII trials, the US Food and Drug Administration has authorized adjuvant therapy for patients with primary, nonmetastatic, high-risk tumors (stageIIB-IIC). The National Comprehensive Cancer Network has already incorporated the recommendation to use pembrolizumab monotherapy in such cases in its clinical practice guidelines (Table 3).10,31 This indication is not authorized in Spain.
In the more recent field of neoadjuvant therapy for advanced melanoma, preliminary studies reported that ipilimumab plus nivolumab before lymph node dissection was associated with major pathologic responses in 61% of metastatic lymph nodes, although 22% of patients developed grade 3 to 4 toxicity.32 A very recent phase II trial showed a clear 2-year survival benefit for neoadjuvant plus adjuvant pembrolizumab compared with pembrolizumab only after lymph node dissection (72% vs. 49%, P=.004).33
Decisions on treatment strategies for BRAF-mutant melanoma should also be based on comorbidities and expected drug toxicity. In countries where adjuvant therapy with dabrafenib plus trametinib is authorized, and following the recommendation to use BRAF and MEK inhibitors in patients with low tumor burden, targeted therapy would appear to be the adjuvant treatment of choice for BRAF-mutant melanoma.27
Sequence of Initial Systemic Treatment for Patients with BRAF-Mutant Melanoma and Unresectable MetastasesEvidence derived from indirect comparisons, mechanisms of action, safety profiles, and administration routes to guide decisions on treatment sequencing for BRAF-mutant melanoma was recently complemented by direct evidence from the phase III DREAMseq clinical trial. In this trial, patients with unresectable metastatic BRAF V600-mutant melanoma who had not received previous treatment for metastatic disease or adjuvant therapy were assigned to 2 groups: one that started with ipilimumab plus nivolumab followed by dabrafenib plus trametinib in the event of disease progression, and another that started with dabrafenib plus trametinib followed by ipilimumab plus nivolumab in the event of progression (Fig. 2). Overall 2-year survival rates clearly favored treatment initiation with ipilimumab plus nivolumab (71.8% vs. 51.5%, P=.01). This survival advantage was maintained in all subgroups, even in patients who would supposedly have a better initial response to dabrafenib plus trametinib (ECOG PS = 0, normal LDH levels, and low tumor burden with < 3 metastatic sites).34 Furthermore, the overall objective response rate was similar in patients initiated on ipilimumab plus nivolumab vs. dabrafenib plus trametinib (46.0% vs. 43.0%). However, the switch to dabrafenib plus trametinib at disease progression achieved a similar objective response to that achieved with the first-line treatment (47.8%). Ipilimumab plus nivolumab, by contrast, was less effective than the first-line option when used to treat progression (objective response, 29.6%). These results suggest that ipilimumab plus nivolumab after progression in patients receiving dabrafenib plus trametinib is less effective than first-line treatment.34 Differences in the frequency of grade 3 adverse events were not significant between the 2 sequences, but grade 4 events were more common in patients started on ipilimumab plus nivolumab.34
Treatment sequences compared in the DREAMseq clinical trial of patients with unresectable metastatic BRAF-mutant melanoma.34 IV indicates intravenous.
The above results led the authors to conclude that the optimal sequence for most patients with BRAF-mutant melanoma is ipilimumab plus nivolumab, followed by BRAF and MEK inhibitors if necessary. This may, however, not be applicable to patients previously treated with immunotherapy or patients treated with adjuvant anti-PD1 or anti-CTLA4 immunotherapy or BRAF and MEK inhibitors, as these were excluded from the clinical trial.34 Although comparative clinical trials have not been conducted, indirect long-term comparative data from pivotal trials and clinical registry data may also support initial treatment with anti-PD1 monotherapy.
In the phase II SECOMBIT trial, although no formal comparisons were made, first-line ipilimumab plus nivolumab followed by encorafenib plus binimetinib was associated with the best overall survival at 3 years (62%).35 The best overall response rate for ipilimumab plus nivolumab (57.9%) was observed when the combination was administered using a “sandwich” strategy consisting of encorafenib plus binimetinib for 8weeks followed by nivolumab plus ipilimumab, and, in patients who experienced disease progression, encorafenib plus binimetinib.35 The authors mentioned that this strategy could boost initial responses while maintaining long-term benefits (phaseII EBIN-EORTC trial ClinicalTrials.gov identifier: NCT03235245).
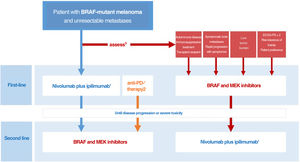
Drawing on the conclusions of the DREAMseq clinical trial, indirect evidence, and prior experience with BRAF and MEK inhibitors, we have designed an algorithm for guiding decisions aimed at tailoring treatment to all concurrent conditions, optimizing survival outcomes, and reducing variability in clinical practice (Fig. 3). This proposal, however, will need to be revised as clinically relevant data from ongoing clinical trials examining different treatment sequences become available.
Decision-making algorithm for first-line systemic treatment of BRAF-mutant cutaneous melanoma. 1.Ipilimumab plus nivolumab should be considered the first-line treatment in most clinical situations. 2.Indirect comparisons from pivotal trials and clinical registry data suggest that anti-PD1 monotherapy with nivolumab or pembrolizumab may offer the same results as initial anti-CTLA-4 plus anti-PD1 immunotherapy. 3.The multidisciplinary committee should consider clinical and progression-related factors that would favor initiation of systemic BRAF and MEK inhibitor therapy. 4.The need for high-dose corticosteroids to treat symptomatic brain metastases is a limiting factor for immunotherapy. 5.Objective parameters indicative of low tumor burden: normal lactate dehydrogenase levels, ≤3metastatic sites, sum of longest diameters of target lesions <44mm.
ECOG-PS indicates Eastern Cooperative Oncology Group performance status.
The authors declare that they have no conflicts of interest.