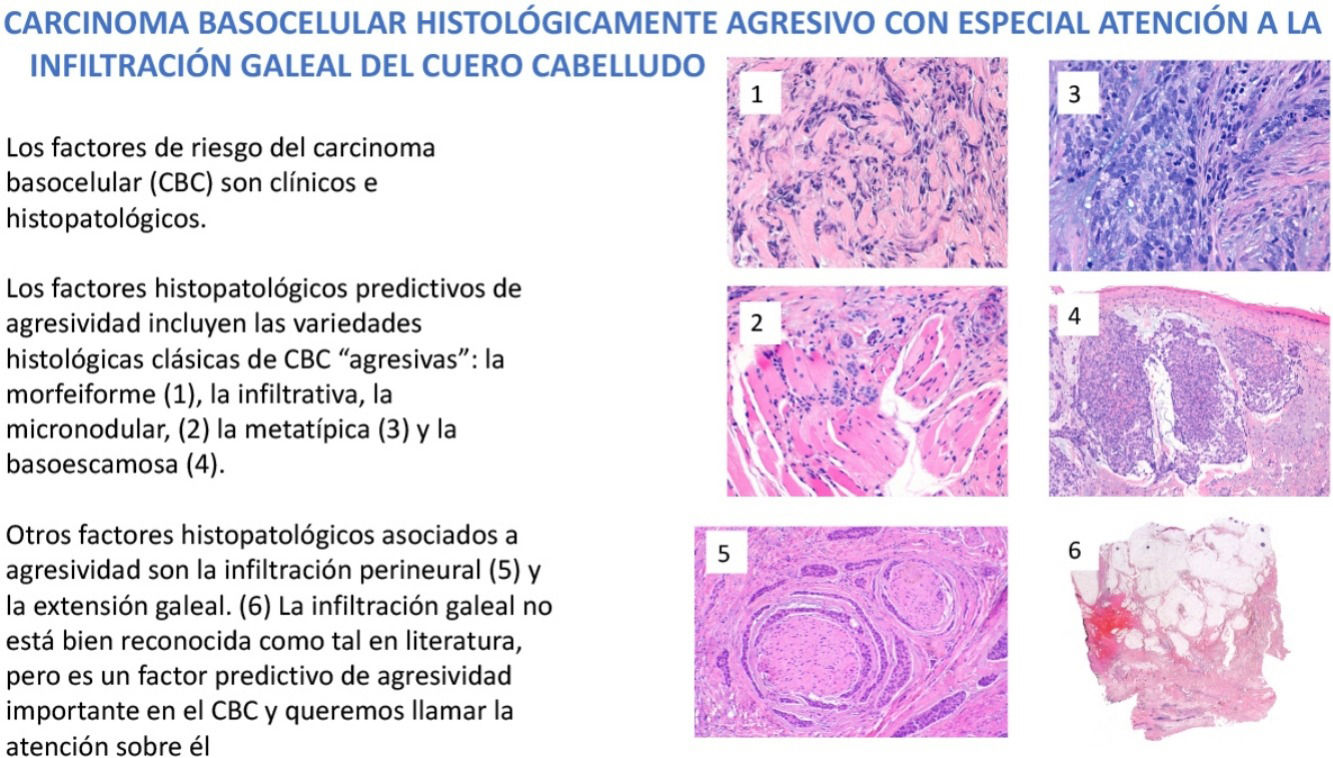
Familiarity with predictors of more aggressive behavior is crucial to the management of basal cell carcinoma (BCC). Risk factors for aggressive BCC are essentially divided into clinical and histopathologic factors. In this review we examine histopathologic features predictive of aggressiveness in BCC. The morpheaform, infiltrative, micronodular, metatypical, and basosquamous subtypes and BCC with sarcomatoid differentiation are classically considered predictive of aggressive behavior. However, 2 other features associated with aggressive BCC are perineural invasion (invasion of nerves below the dermis or nerves larger than 0.1mm in caliber) and subgaleal extension. While the former is well known and widely described in the literature, the latter is not generally recognized as a risk factor, even though it is predictive of highly aggressive behavior. In this review, we draw attention to its importance.
En el manejo del carcinoma basocelular (CBC) es fundamental conocer los factores de riesgo que predicen un comportamiento más agresivo. Estos factores de riesgo se dividen esencialmente en factores clínicos y en factores histopatológicos. En este trabajo revisamos e ilustramos los hallazgos histopatológicos predictivos de agresividad en el CBC. Dichos factores histopatológicos predictivos de agresividad incluyen las variedades histológicas clásicas de CBC «agresivas»: la morfeiforme, la infiltrativa, la micronodular, la metatípica, la basoescamosa y el CBC con diferenciación sarcomatoide. Pero, aparte de las variedades referidas, existen también 2 hallazgos histopatológicos asociados a CBC agresivos, uno bien conocido y reflejado en la literatura, que es la infiltración perineural de filetes nerviosos de más de 0,1mm de diámetro o subdérmicos, y el otro es la extensión subgaleal. La infiltración galeal no está bien reconocida como tal en literatura, pero es un factor predictivo de agresividad importante en el CBC y queremos llamar la atención sobre él.
Basal cell carcinoma (BCC) is the most frequent type of cancer in humans. Although its global incidence is difficult to calculate, according to the most recent estimates, the annual incidence in the United States between 1998 and 2021 was approximately 2 million cases,1–3 which would correspond to 10 million new cases of BCC a year throughout the world. Although the behavior of this tumor is generally not very aggressive—fewer than 1% of all cases lead to mutilating locoregional destruction and/or metastases and a fatal outcome is very rare—the huge global incidence means that aggressive cases should not be ignored.
The problem with BCCs that follow an aggressive local course or undergo metastasis is often that the aggressive potential is underestimated not only by the patient but also, unfortunately, by the treating physician, including the dermatologist.
Dermatologists should be familiar with the risk factors predictive of a possible more aggressive behavior of some BCCs. These risk factors are divided essentially into clinical factors and histologic ones. Among the clinical risk factors in BCC, we highlight location, with a series of high-risk (H) locations indicated by the National Comprehensive Cancer Network (NCCN) and that include the central face, periocular and periauricular locations, and genitalia and the hands and feet. It is interesting to note that the scalp, recognized in the 2 classic reviews of metastatic BCC as an overrepresented region in this group of aggressive BCCs,4,5 is not included as a high-risk location in the guidelines or in most texts. In contrast, the scalp is usually considered as a medium (M) risk location for BCC. Other clinical risk factors for aggressive BCC are longstanding duration, cases neglected by the patient or physician, cases of multiple recurrence, size greater than 5cm (giant BCCs), depth of invasion, and, of particular weight, a history of prior radiotherapy of the affected area. In this review, we will pay particular attention to the histopathologic features predictive of aggressive behavior in BCC and cover the classic aggressive histologic variants of BCC: morpheaform, infiltrative, micronodular, metatypical, and basosquamous variants, and variants with presence of sarcomatoid differentiation given the greater tendency of such tumors to local infiltration and their association with recurrent, locally advanced, and/or rare cases of metastatic BCC. We will also review 2 histologic features. The first is well documented in the literature and possibly afforded too much importance, as explained later, namely perineural invasion of nerve fibers either greater than 0.1mm in caliber or at a location below the dermis. The second is subgaleal extension, which has not been recognized in the literature as a predictive factor for aggressive BCC and which we would like to draw attention to.
Although dermatologists tend to believe that we are able to largely predict tumors that correspond to an aggressive histological variant through clinical examination, a recent prospective multicenter French study of 2274 cases demonstrated that presurgical diagnosis of histologically aggressive forms (which they took to be synonymous with morpheaform patterns) was only correct in 22% of cases.7 On the other hand, we should not forget that it is estimated that up to 20% of cases with prior punch biopsy of BCC can be mistaken in the determination of the predominant histological subtype with respect to study of the completely excised tumor. This is partly explained because around 40–70% of BCCs are not homogeneous but mixed with different patterns coexisting in the same tumor.
Aggressive histological variants in basal cell carcinomaThere are more than 20 histological variants of BCCS reported: superficial, solid or nodular, morpheaform, infiltrative, micronodular, metatypical, basosquamous, with squamous differentiation, keratinizing, adenoid, cystic, pigment, keloidal, infundibulocystic, fibroepithelioma of Pinkus, with sebaceous differentiation, with matrical differentiation, granular cell, with monster cells, signet ring, adamantinoid, with Schwann cells, among others. However, 95% of all BCCS belong to one of the 5 most frequent variants: nodular (or solid) accounting for about 70% of cases, superficial (10–15%), morpheaform (5%), infiltrative (5%), and micronodular (5%).
To determine which variants of BCC can be considered as aggressive, we highlight the largest review published to date of metastatic BCC, which found that the morpheaform and infiltrative variants accounted for 19% of cases of metastasis.6 More striking is the case of metatypical and basosquamous BCCs, which accounted for 38% of all cases of metastasis; this gives an idea of their potential for aggressive behavior given that the 2 variants make up at most 3% of all BCC.
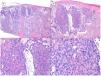
There is a lot of confusion in the literature regarding the terms metatypical and basosquamous in reference to BCC variants. Many authors, including classic dermatopathology texts such as the one by Mckee, consider these terms synonymous and use them indistinctly.7 However, other authors, including also classic dermatopathology texts such as the one by Weedon, make a clear distinction between them. Thus, both metatypical BCC and basosquamous BCC share the feature of absence of a row of columnar cells at the periphery of their islets and they may or may not have areas of conventional BCC. However, basosquamous BCC shows areas with cells that are almost indistinguishable from squamous cell carcinoma cells (Fig. 1). This feature is not present in metatypical BCC. Metatypical BCC is characterized by the presence of aggregates of metatypical cells (Fig. 2), which are paler and larger cells than those of conventional BCC, but smaller and less eosinophilic than those of squamous cell carcinoma.8 Thus, we could define metatypical BCC as a de-differentiated BCC which, morphologically, resembles Merkel cell carcinoma, whereas basosquamous BCC corresponds to a differentiated BCC with resemblance to squamous cell carcinoma. However, it seems that the distinction between these 2 variants of BCC does not have particularly great practical repercussions as the biological behavior in terms of recurrence and survival is similar.8 Probably, for this reason, the 2 variants are unified under the term basosquamous carcinoma in the most recent WHO publication on skin tumors.9 The metatypical BCC variant has been described in the literature in cases of BCC treated previously with vismodegib,10 and we have also recently observed this in patients treated with sonidegib (unpublished data).
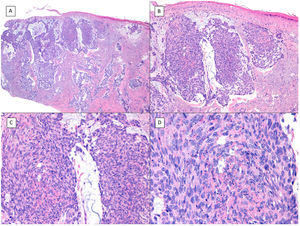
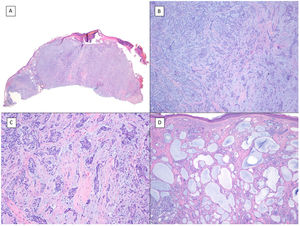
Basosquamous carcinoma (hematoxylin and eosin). A, At low magnification, areas of infiltrative BCC with basaloid nests can be seen in the deep part of the lesion, whereas areas with squamous differentiation are observed in the superficial part (×40). B, In these nests, we do not observe the typical row of columnar cells at the periphery of the islets as is the case in conventional BCC (×100). C, Tumor cells show eosinophilic staining and a spindle-cell morphology, more reminiscent of squamous cell carcinoma (×200). D, Detail of the squamous-like cells (×400).
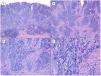
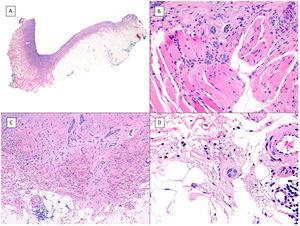
Metatypical pattern of BCC with partial response after treatment with sonidegib (hematoxylin and eosin). A, In the low-magnification image of the punch biopsy, BCC can be seen in the upper part of the image, with marked inflammatory reaction at the deep dermal margin of the tumor (×40). B, Nests of undifferentiated BCC with desmoplastic stromal reaction with abundant mucin (×100). C, These nests of metatypical BCC lack peripheral palisading and there are no retraction clefts between the epithelium and the stroma (×200). D, The high-magnification image shows the typical appearance of metatypical cells, which are less basophilic and larger than cells in conventional BCC and less eosinophilic and smaller than those of squamous cell carcinoma (×400).
The most important differential diagnoses for these 2 variants of BCC in terms of practical repercussions are squamous cell carcinoma in the case of basosquamous BCC and Merkel cell carcinoma in the case of metatypical BCC. In case of doubt, immunohistochemical study is useful given that squamous cell carcinoma is negative for BER-Ep4 whereas BCC is positive, and Merkel cell carcinoma is positive for CK20 but negative in BCC.
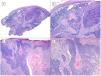
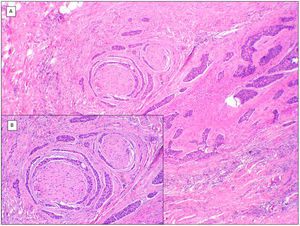
There are another 2 terms that can give rise to confusion with the previous 2 and that may be present in a pathology report, namely, BCC with squamous differentiation and keratinizing BCC (Fig. 3). These latter 2 BCC variants have all the characteristics of conventional BCC, but one has islets of squamous cell carcinoma within the tumor while the latter has abrupt keratinization of islets in the second.8 Neither of these 2 variants of BCC appear to be have a more aggressive behavior.
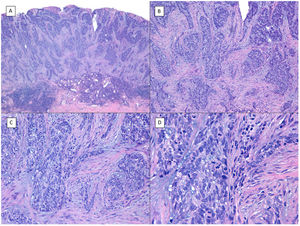
BCCs with squamous cell differentiation (A, B) and keratinization (C, D) (hematoxylin and eosin). A, In BCC with squamous cell differentiation, a predominance of conventional nodular BCC can be observed at low magnification (×40). B, Focally, 2 well differentiated squamous areas can be seen in one of the typically basaloid tumor nests (×100). C, In keratinizing BCC, conventional nests of nodular BCC predominate with areas of necrosis on the right side (×100). D, At higher magnification, 2 foci of abrupt keratinization can be seen in one of the islets (×200).
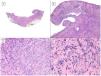
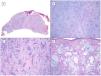
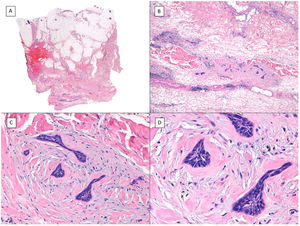
Another variant traditionally considered as aggressive is the morpheaform or sclerosing variant. This variant is characterized by prominent fibrosis, occasionally almost keloid, of the stroma, and by thin and/or small nests of basaloid cells without peripheral palisading or retraction clefts with the stroma (Fig. 4). This variant often affects the deep dermis and perineural invasion is not uncommon. Clinically, it usually corresponds to scar-like plaques although this observation may be deceptive.7
Infiltrative/morpheaform pattern (hematoxylin and eosin). A, In the debulking visualization prior to Mohs surgery, a tumor is seen invading deep layers, with massive involvement of the hypodermis (×10). B, In the most superficial part of the tumor, there are areas of conventional nodular BCC (×40). C, In deep areas of the same tumor, the invasive and morpheaform pattern predominates. These areas show how tumor cells are arranged in rows of narrow and elongated nests embedded in a densely collagenous stroma (×200). D, Detail of the morpheaform pattern in which dense collagen can be seen in thick, almost keloid bands (×400).
The infiltrative BCC variant resembles the morpheaform variant in that there are elongated nests, which can sometimes be very small, without forming a row, palisading, or clefts between epithelial islets and the stroma, but it is not associated with fibrotic stroma seen in morpheaform BCC. Often, infiltrative BCC is associated with areas of morpheaform BCC or conventional superficial nodular variants in others. This may lead to underassessment in the initial biopsy if the biopsy is only superficial. Of interest is that the presence of mixed histological patterns (Fig. 5) has been associated in some reports with an increased probability of subclinical spread.11,12
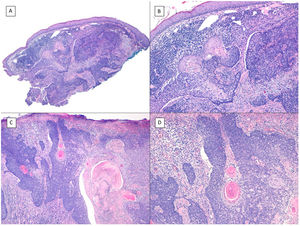
Mixed pattern (hematoxylin and eosin). A, BCC with a predominantly invasive and morpheaform pattern and adenoid areas in the left half of the tumor (×10). B, Infiltrative/morpheaform pattern with small and elongated epithelial islets (×100). C, Detail of the morpheaform pattern with invasive epithelial nests and desmoplastic stromal reaction (×200). D, Detail of the adenoid part of the same tumor, with cystic spaces filled with mucin (×200).
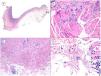
Another aggressive BCC deserving of mention is the micronodular variant, which is a peculiar BCC variant consisting of less than 50% of small rounded palisaded epithelial basaloid islands without rows at the periphery of the island or retraction clefts between the epithelium and the stroma. This BCC variant has a much higher potential for histologic invasion than suggested by clinical observations and, in our experience, is often located in the perinasal area. The micronodular BCC islands are usually little cohesive at the tumor periphery (Fig. 6), such that this, in combination with the small size of these islands (less than 0.15mm), can make it difficult to obtain reliable tumor free margins, even with frozen sections in Mohs surgery.
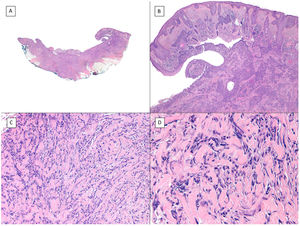
Micronodular pattern (hematoxylin and eosin). A, BCC located in the nasogenian fold and cheek with a micronodular pattern that predominantly affects the dermis with points of extension to the hypodermis and striated muscle (×10). B, Detail of the small and rounded nests typical of this variant of BCC invading the striated muscle (×200). C, Image of extension toward the hypodermis of the same tumor (×100). D, Detail of (C) in which a small nest can be seen distant from the primary tumor, demonstrating the incohesive nature of the periphery of micronodular BCC (×400).
The last aggressive variant is the sarcomatoid variant, in which the mesenchymal component of BCC consists of a pleomorphic sarcoma, an osteosarcoma, a chondrosarcoma, a leiomyosarcoma, and/or a rhabdomyosarcoma. It appears that the epithelial component (BCC) and the mesenchymal component (sarcoma) have a similar profile of chromosomal changes, an observation that supports a common but divergent origin of the 2 components.
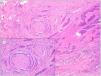
Other histologic features predictive of aggressive behavior: perineural invasion and subgaleal extensionPerineural (Fig. 7) and subgaleal invasion (Fig. 8) by BCC implies occupation and invasion of a virtual space by tumor cells: the perineural region in the first case and the subgaleal space in the second. Usually, these tumor cells tend to occupy these “virtual spaces” such that they expand them and can be recognized in histological sections. However, at certain locations, and in unpredictable fashion, the cancer cells pass through these spaces without expanding them. These sites are known as skip areas, and they can go undetected if the histological section coincides with these areas. This implies that the tumor cells with this route of invasion have the capacity to spread beyond where we are able to detect them. This could explain the greater potential for aggressive behavior given their frequent subclinical spread and the difficulty to achieve complete excision by conventional surgery. The incidence of perineural invasion in BCC is between 0.17% and 2.74% across all variants of BCC. However, most BCC with perineural invasion are asymptomatic lesions and they are considered an incidental finding when observed under the microscope, particularly when they affect nerves below the dermis or those nerves smaller than 0.1mm in caliber. Perineural invasion is associated with aggressive variants of BCC, particularly if the tumor is located on the face and particularly at sites contiguous to the ear, with recurrent tumors, and if patients have received prior radiotherapy. Perineural invasion is considered a risk factor in BCC, such that the presence of perineural invasion of nerves below the dermis or those larger than 0.1mm in caliber is one of the 5 minimum features required in the pathology report for BCC according to the NCCN. However, a recent study has shown that perineural invasion of unnamed nerves has no impact on the behavior of BCC.13 Likewise, another recent study has shown that whether perineural invasion is a marker of aggressive BCC depends on the presence of other factors of poor prognosis; thus, although associated with an aggressive course in the univariate analysis, this association is lost in the multivariate analysis.14 Should future studies confirm these findings, perineural invasion will likely lose much of its weight as a histological feature predictive of an aggressive course in BCC.
Subgaleal invasion (hematoxylin and eosin). A, BCC apparent only in the deepest part of the image; the entire dermis and hypodermis in most of the sections of this same tumor are disease-free (×10). B, The basaloid nests invade the subgaleal plane with no connection to the underlying planes (×100). C, Each of the epithelial nests is surrounded by dense and thickened collagen (×200). D, Detail of the tumor nests (×400).
With regards subgaleal extension of BCC in the scalp, we believe opposite is true, that is, the potential for an aggressive course is underestimated. In effect, in our hospital, which is a referral center for cancer, we have found that the tumor margins are hard to predict in BCC with this type of histologically confirmed extension, and full resection often requires many stages of Mohs surgery (we have experienced several cases of up to 7 and 10 stages with these characteristics). Even in cases of mutilating surgery in which Mohs surgery had to be continued for several consecutive days, we have achieved BCC with supposed negative margins and subsequent recurrence (unpublished data). Moreover, as already mentioned, in reviews published of all metastatic BBC between 1894 and 2011,4,5 of note is that the scalp is one of the most frequent sites in these cases. Unfortunately, in these reviews, there is no description of the degree of scalp invasion by BCC. Of note is that the site most closely associated with the origin of the metastatic cases is considered as the “M zone” (location with moderate risk of recurrence) in all BCC guidelines. This leads to the hypothesis that this particular type of invasion (subgaleal), although uncommon in BCC of the scalp, is the origin of the striking aggressive course of BCC at this site in large series. According to this hypothesis, most BCCs of the scalp correspond to cases without subgaleal extension and are therefore classified as “M zone,” but in cases with subgaleal spread they could potentially be considered as “H zone” (that is, a location with high risk of recurrence). Another explanation for the more aggressive behavior of scalp BCC proposed by some authors is the delay in diagnosis because of hair coverage, in conjunction with the abundance of lymph and blood vessels at this site.15 However, in our experience, cases with more aggressive locoregional behavior correspond to BCC that has spread in the subgaleal plane, and so we believe that while these factors have some impact, they are not as important as subgaleal extension.
Conflict of interestThe authors declare that they have no conflict of interest.