Meglumine antimoniate (MA) (Glucantime®, Sanofi-Aventis, S.A., Spain) is the treatment of choice in cutaneous leishmaniasis (CL).1–4 It is administered intramuscularly (IM) and intralesionally (IL), and is considered to be a safe and effective drug. IL administration is used in single lesions smaller than 3–4cm and IM administration is reserved for multiple lesions, complicated lesions, or cases with signs of lymphatic dissemination.1,3,4 The recommended IM dosage is between 10 and 20mg/kg/d of antimony (75mg/kg/d of MA) in 10-day cycles.3,5–7 For IL administration, much lower doses of between 0.2 and 1mL/lesion are used, in variable dosage regimens, every 0.5-1-2 weeks.1,3,8 It is well known that systemic use of the drug may cause electrocardiographic abnormalities.3
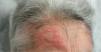
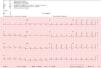
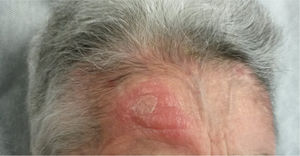
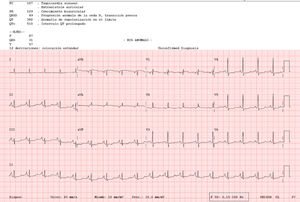
An 82-year-old woman visited our department with an erythematous–edematous infiltrated lesion measuring 3.5cm, with no ulceration or scab, located on the forehead; the lesion was persistent and had appeared some months earlier (Fig. 1). The patient had a past history of rheumatoid arthritis treated with etanercept and prednisone, osteoporosis, scoliosis, mild anemia, seborrheic dermatitis treated with topical corticosteroids, and severe underweight (BMI, 15kg/m2). A skin biopsy of the lesion revealed granulomatous dermatitis with amastigotes and a diagnosis of leishmaniasis was therefore established. The patient had not traveled outside Spain. It was decided to treat the patient with oral itraconazole for 6 weeks, with good clinical and analytical tolerance but without improvement. A week later, intralesional MA was administered at a dose of 0.6–1mL on days 0, 7, and 21, following administration of lidocaine/prilocaine. The topical corticosteroids and etanercept were withdrawn from the moment of diagnosis. An electrocardiogram (ECG) performed after the 3rd dose detected a prolonged QT interval (QTc, 510ms), nonsignificant ST segment depression, and increased P wave axis (Fig. 2), and treatment was therefore suspended. The patient reported no episodes of syncope. A follow-up ECG, performed by the cardiology department 3 weeks later, was within the normal range. The cutaneous signs and symptoms resolved with the 3 doses that had already been administered.
Multiple adverse effects of systemic MA have been reported, including myalgia, arthralgia, gastrointestinal disorders (nausea, abdominal pain), headache, elevated hepatic and pancreatic enzymes, leukopenia, abnormal ECG, and severe arhythmia.3,5 Because it involves lower and more widely spaced doses, intralesional use of MA produces mild and generally local adverse effects (pain, edema, pruritus, and transitory erythema at the injection site).2,4,5,7–10 Systemic adverse effects associated with this route of administration have also been described, such as nausea, vomiting, dyspnea, dizziness, rash, myalgia, arthralgia, headache, and even anaphylactic shock.10 Cardiotoxicity due to systemic antimonials is a well-known adverse effect that, according to the product information sheet, may present when used at high daily doses over long periods of time. It may produce a prolonged QT interval in the ECG, with potential development of severe arrhythmia, which may result in death. Changes in the ECG are generally dose-dependent and are usually reversible. In most cases, abnormalities such as T wave inversion and prolonged QT interval precede onset and are a warning sign of potential severe arrhythmia. A baseline ECG should be performed with a follow-up ECG every 7–10 days, and treatment should be suspended if the QTc interval exceeds 450ms.5,6,9 Ribeiro et al. also demonstrated changes in the ECG (prolonged QT) with systemic IM therapy at low doses (10mg/kg) and in short treatment durations (10 days) with potential severe implications, which make monitoring these patients using ECG recommendable.6 With regard to intralesional therapy, a recent clinical study in Brazil in 53 patients who underwent a weekly ECG found a prolonged QT interval with no clinical repercussions in 25% of cases.10 This effect was found to be associated with smoking.10 It is, however, a poorly documented adverse effect, as ECGs are not usually performed in the clinical follow-up of intralesional therapy. In that study, the weekly doses used were much higher than those in our study. The fact that we detected this abnormality in a patient with no cardiologic risk criteria in treatment with low-dose intralesional MA leads us to believe that it is also useful to monitor the ECG in these patients.
In conclusion, before instating therapy with antimonials in any form, the patient should be questioned regarding personal history of cardiac disease (heart attack, bradycardia, palpitations, syncope, etc.) and a family history of sudden death. Factors that may favor prolonged QT, such as electrolyte imbalance, should be monitored and corrected and association with other drugs that may also cause prolonged QT (www.qtdrugs.org) (antiarrhythmics, tricyclic antidepressants, erythromycin, tetracyclines, trimethoprim/sulfamethoxazole, antipsychotics, etc.) should be avoided.6 We recommend monitoring high-risk patients and watching for the appearance of a prolonged QT interval.
Conflicts of InterestThe authors declare that they have no conflicts of interest.