Genital warts are caused by the human papillomavirus (HPV), whose genotypes have traditionally been classified as low risk or high risk (oncogenic). The first 2 prophylactic vaccines included the most common genotypes at the time: HPV-6, HPV-11, HPV-16, and HPV-18. The aim of this study was to evaluate the prevalence of HPV types in our setting 10 years after the introduction of HPV vaccines.
Material and methodsDescriptive, observational, retrospective study of patients diagnosed with genital warts at the sexually transmitted infection unit of a dermatology department between January 2016 and June 2019.
ResultsIn total, 362 patients were diagnosed with genital warts during the study period, and 212 (58.6%) underwent genotyping. Thirty-two distinct HPV types were observed, the most common being HPV-6, HPV-11, HPV-16, and HPV-42. HPV DNA was detected in 93.9% of the samples analyzed, and there were 299 genotypes (mean, 1.5 per patient). Overall, 26.6% of patients had more than a single HPV genotype, while 24.1% had at least 1 high-risk type. No significant associations were found between the presence of high-risk HPV types and any of the study variables. At least 2 of the 4 HPV types targeted in the original vaccines were detected in 94.1% of lesions.
ConclusionsCompared to 10 years ago, the prevalences of HPV types included in the first 2 prophylactic vaccines have decreased, while the proportion of patients with at least 1 of the 4 most common types has remained unchanged. We also observed a slight increase in infections with multiple HPV types or at least 1 high-risk type.
El condiloma acuminado está causado por el virus del papiloma humano (VPH), cuyos genotipos se han descrito tradicionalmente como de bajo y alto riesgo (AR) oncogénico. Clásicamente, los genotipos más frecuentes son el 6, el 11, el 16 y el 18, incluidos en las dos primeras vacunas desarrolladas. Nuestro objetivo es valorar cambios en la prevalencia de estos genotipos tras 10 años desde la instauración de la vacuna profiláctica en nuestro medio.
Material y métodosSe trata de un estudio observacional descriptivo retrospectivo realizado en la Unidad de Infecciones de Transmisión Sexual (UITS) de un Servicio de Dermatología entre enero de 2016 y junio de 2019, seleccionando posteriormente a los pacientes diagnosticados de condilomas acuminados.
ResultadosSe han diagnosticado 362 pacientes con condilomas acuminados, realizándose genotipado en 212 pacientes (58,6%). Se han detectado 32 genotipos distintos, siendo los más frecuentes el 6, el 11, el 16 y el 42. En el 93,9%, la detección de VPH fue positiva, detectándose hasta 299 genotipos, lo que corresponde a 1,5 por paciente. En el 26,6% de pacientes se detectaron más de un genotipo distinto de VPH. En el 24,1% se detectó al menos un genotipo de AR. No se observó asociación estadísticamente significativa entre la presencia de un genotipo de AR y las variables estudiadas. En el 91,4% de las lesiones se aisló al menos uno de los cuatro genotipos cubiertos por las dos primeras vacunas desarrolladas.
ConclusionesLa prevalencia de los genotipos de VPH incluidos en las dos primeras vacunas profilácticas desarrolladas ha disminuido. La implicación de al menos uno de los cuatro genotipos más frecuentes se ha mantenido estable con respecto a hace 10 años. Las infecciones por múltiples genotipos y la presencia de al menos un genotipo de AR oncogénico ha aumentado ligeramente.
Genital warts (condyloma acuminata) are the most common sexually transmitted infection (STI) in the world.1 According to estimates, approximately 5%–10% of the population will experience at least 1 episode during their lifetime.2 Genital warts are caused by the human papillomavirus (HPV), which is a member of the Papillomaviridae family. Papillomaviruses have traditionally been described as genotypes, as they are classified according to the degree of sequence homology with the DNA of the viruses. Genotypes are identified using numbers following the historical order in which they were described. More than 150 genotypes have been detected to date, and approximately 40 of these can cause genital infections. HPV genotypes are classified according to their oncogenic potential into low-risk types, which essentially cause genital warts, and high-risk types, which are frequently associated with invasive cervical carcinoma and other malignant tumors in the genital and oropharyngeal areas.3,4 There are 18 high-risk types (HPV-16, -18, -26, -31, -33, -35, -39, -45, -51, -52, -53, -56, -58, -59, -66, -68, -73, and -82) and 16 low-risk types (HPV-6, -11, -40, -42, -43, -44, -54, -61, -67, -69, -70, -71, -72, -81, -84, and -89). Those that do not fall into either category are classified as undetermined-risk types.5 High-risk types can primarily be differentiated from other types by the structure and function of E6 and E7 gene products. In benign lesions, viral DNA is present in episomal form rather than integrated into the host cell genome, as occurs with malignant lesions.6
HPV-6, HPV-11, HPV-16, and HPV-18 were targeted by the first 2 HPV vaccines developed, as they have traditionally been the main genotypes detected in the tissue of genital warts,7 independently of geographic area, sex,5 or HIV status.8 High-risk genotypes have been identified in up to 35% of genital warts9 and are particularly common in women.10 A doctoral study conducted by Hernández-Bel1 in 2007 and 2008 found that HPV-6, HPV-11, HPV-16, HPV-18, and HPV-81 were, in decreasing order of frequency, the 5 most common types in our setting, and were present in 90% of lesions. High-risk genotypes were detected in 20% of genital warts.
Routine screening for HPV genotypes is not recommended in the case of genital warts, as it does not alter the clinical picture or treatment. It is only used in cases of doubtful diagnosis or to check for the presence of HPV in children when sexual abuse is suspected.
Prophylactic vaccination has emerged as an important strategy for preventing HPV infection, and consequently, precancerous lesions.11 A sine qua non condition for the successful prevention of HPV-related cancer is vaccination before the first sexual contact. Three HPV vaccines are currently authorized for use in Spain. The first 2 vaccines developed, both bivalent, target HPV-16 and HPV-18.12 The third one is quadrivalent and targets HPV-6, HPV-11, HPV-16, and HPV-18. By imparting immunity to the low-risk types responsible for most genital warts, this last vaccine (Gardasil) is capable of reducing the incidence of these lesions.2 The appearance of Gardasil-9, a nonavalent vaccine, in 2017, provided additional protection against HPV-31, HPV-33, HPV-45, HPV-52, and HPV-58. The HPV vaccination program in Spain only contemplates vaccination for girls, and to date, none of the country's autonomous communities have considered vaccinating boys.
The aim of this study was to analyze changes in the prevalence of HPV genotypes present in genital warts more than 10 years after the introduction of HPV vaccines in our setting. A secondary aim was to determine whether the prevalence of high-risk genotypes has decreased in this time and to analyze the association between these types and a range of study variables.
Material and MethodsWe designed an ambispective observational study in which we collected retrospective and prospective data from consecutive patients seen at the sexually transmitted infection (STI) unit of the dermatology department at Consorcio Hospital General Universitario de Valencia (CHGUV) in Valencia, Spain, between January 2016 and June 2019. The STI unit provides services to patients from the hospital's catchment area and beyond. The patients come from multiple sources, including referrals for dermatology consultation in the emergency department on any day of the year and referrals from primary care centers attached to the hospital, dermatologists in the area, and other hospital departments, in particular, infectious diseases, gynecology, and urology. All patients seen during this period who were diagnosed with genital warts were included in this study.
The STI unit at our hospital has been performing routine HPV genotyping in consenting patients with genital warts for diagnostic and research purposes for over 10 years. Tissue samples obtained by biopsy are divided into 2 parts: one for histologic examination with hematoxylin-eosin staining and the other for HPV genotyping using HPV Direct Flow CHIP. This is an in vitro kit that allows the qualitative detection and typing of 36 HPV genotypes via amplification of a fragment of the papillomavirus L1 region by polymerase chain reaction followed by reverse hybridization on a membrane containing specific probes. With this protocol, clinical samples can also be amplified directly, without prior DNA extraction.
We collected epidemiological data (sex, age, nationality, sexual orientation, reason for consultation, patient origin, history of previous STIs, and previous HPV vaccination) and clinical data (location of warts, genotypes detected, and internal involvement evaluated by rectoscopy). The information was collected through an open oral interview and a standardized questionnaire previously authorized by the hospital's clinical research ethics committee.
Results were analyzed using descriptive statistics, with means (SD) for quantitative variables and percentages for qualitative variables. Comparisons were made with the χ2 or Fisher exact test, as appropriate. Statistical data were analyzed in SPSS version 21.0 (IBM Corp., Armonk, NY). A significance level of P<.05 was established.
ResultsBetween January 2016 and June 2019, 1181 patients with suspected venereal disease were seen at the CHGUV STI unit; there were 1254 first visits and 1003 diagnoses of venereal disease. None of the patients were transgender; 702 (70%) of the diagnoses corresponded to cisgender men and 301 (30%) to cisgender women.
In total, 362 diagnoses of genital warts were made (36.8% of all STIs diagnosed). There were 221 men (61%) with a mean age of 36.5 years and 141 women (39%) with a mean age of 34.6 years. The differences were not significant. Twenty-nine patients (8%) were aged 20 years or younger, 115 (31.8%) 21 to 30 years, 106 (29.3%) 31 to 40 years, 75 (20.7%) 41 to 50 years, and 37 (10.2%) 51 years or older. In total, 285 patients (83.5%) were heterosexual, 313 (86.5%) were Spanish, and 58 (16.2%) were HIV-positive. No significant differences in mean age were observed between men and women (35 vs. 34 years, P=.3) or between heterosexual and homosexual people (35 vs. 33 years, P=.2).
HPV genotyping was performed in 212 patients (58.6%), and 13 samples (6.1%) were negative. Thirty-two distinct genotypes were detected; 199 patients (93.9%) had a positive test and 299 genotypes were detected (mean, 1.5 per patient). Fifty-three patients (26.6%) had more than 1 genotype, while 48 (24.1%) had at least 1 high-risk genotype. The results are summarized in Table 1, with a distinction between low- and high-risk types. To facilitate statistical analysis and reporting, intermediate-risk types were included in the low-risk category.
Prevalence of HPV Infection in Genital Warts According to Low- Versus High-risk Classification.
| No. (%) of patients | P valueb | |||
|---|---|---|---|---|
| Men (n=118) | Women (n=81) | Total (n=199) | ||
| No. of different genotypes | ||||
| 1 | 93 (78.8) | 53 (65.4) | 146 (73.4) | |
| 2 | 14 (11.9) | 10 (12.3) | 24 (12.1) | .7 |
| 3 | 6 (5.1) | 11 (13.6) | 17 (8.5) | |
| 4 | 3 (2.5) | 4 (4.9) | 7 (3.5) | |
| 5 | 2 (1.7) | 2 (2.4) | 4 (2) | |
| 6 | 0 (0) | 1 (1.4) | 1 (0.5) | |
| ≥2 | 25 (21.1) | 28 (34.5) | 53 (26.6) | .04 |
| ≥1 low-risk genotypesa (no high-risk types) | 96 (81.3) | 54 (66.7) | 150 (75.4) | .04 |
| ≥1 high-risk genotype (no low- or intermediate-risk types) | 4 (3.4) | 4 (4.9) | 8 (4) | .4 |
| Low-riska and high-risk types | 20 (16.9) | 20 (24.7) | 40 (20.1) | 1.0 |
| HPV-6/HPV-11 | 109 (92.4) | 64 (79) | 173 (86.9) | .04 |
| HPV-16/HPV-18 | 9 (7.6) | 10 (12.3) | 19 (9.5) | .3 |
| HPV-6/HPV-11 HPV-16/HPV-18 | 113 (95.7) | 69 (85.2) | 182 (91.4) | .06 |
| HPV-6/HPV-11 HPV-16/HPV-18 only | 93 (78.8) | 48 (59.2) | 141 (70.8) | .01 |
Abbreviation: HPV, human papillomavirus.
No significant associations were observed between the presence of high-risk HPV types and sex, mean age, sexual orientation, nationality, lesion location, history of genital warts, or presence of HIV (Table 2).
Study Variables Associated with High-Risk HPV Genotypes.
| Variable | No. (%) of patients | P value | |
|---|---|---|---|
| High risk (n=48) | Not high risk (n=151) | ||
| Sex | |||
| Male | 23 (47.9) | 96 (63.6) | 0.06 |
| Female | 25 (52.1) | 55 (36.4) | |
| Mean (SD) age, y | 35.3 (11.8) | 35.2 (11.5) | 1.0 |
| Age<30 y | |||
| Yes | 19 (39.6) | 60 (39.5) | 0.1 |
| No | 29 (60.4) | 91 (60.5) | |
| Age<40 y | |||
| Yes | 32 (66.7) | 102 (67.1) | 0.1 |
| No | 16 (33.3) | 49 (32.9) | |
| Sexual orientation | |||
| Heterosexual | 39 (81.3) | 119 (78.8) | 1.0 |
| Homosexual | 9 (18.7) | 29 (19.2) | |
| Nationality | |||
| Spanish | 43 (89.6) | 129 (85.4) | 0.6 |
| Other | 5 (10.4) | 22 (14.6) | |
| Location of lesions | |||
| Anal/perianal | 20 (41.7) | 71 (47) | |
| Other | 28 (58.3) | 80 (53) | 0.5 |
| Previous STI | |||
| Yes | 22 (45.8) | 58 (38.4) | 0.5 |
| No | 26 (54.2) | 87 (57.6) | |
| Presence of HIV | |||
| Yes | 9 (18.7) | 26 (17.2) | 1.0 |
| No | 39 (81.3) | 123 (82.8) | |
| Past history of genital warts | |||
| Yes | 6 (12.5) | 22 (14.6) | 0.4 |
Abbreviations: HPV, human papillomavirus; STI, sexually transmitted infection.
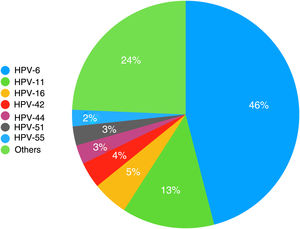
The HPV types detected are shown in order of decreasing frequency in Fig. 1. In addition, HPV-31, HPV-52, and HPV-73 were detected in 6 patients (3%) and HPV-18 and HPV-62 in 5 patients (2.5%). There were 4 cases each of HPV-43, HPV-68, and HPV-81 (2%); 3 cases each of HPV-35, HPV-39, HPV-45, and HPV-54 (1.5%); 2 cases each of HPV-40, HPV-53, HPV-56, HPV-61, HPV-66, HPV-72, and HPV-82 (1%); and 1 case each of HPV-2, HPV-31, HPV-58, HPV-67, HPV-70, and HPV-84 (0.5%).
None of the 221 men diagnosed with genital warts had received prophylactic HPV vaccination in childhood, whereas 14 of the 141 women (9.9%) had. A single genotype was detected in 5 women who had received the Cervarix vaccine: HPV-6 (2 cases), HPV-11 (2 cases), and HPV-40 (1 case). One women, also vaccinated with Cervarix, had 4 genotypes (HPV-6, HPV-39, HPV-51, and HPV-73), while another had 3 (HPV-11, HPV-42, and HPV-67). The vaccine used was unknown in 5 patients; the other 2 had received Cervarix, but the genotyping results were negative.
DiscussionGenital warts are the most prevalent STI in our setting, accounting for 36.8% of all diagnoses. Our findings are consistent with those of the Catalan Health Institute's STI unit.13 We understand that the characteristics of a dedicated hospital-based STI unit may influence the type of patients seen and that these may differ from those at units that treat acute diseases, such as the multidisciplinary clinic specializing in STIs at Hospital Clínic de Barcelona.14
Although men outnumbered women in our study of HPV genotypes in genital warts, they accounted for just 31% of all men diagnosed with an STI, compared with 50% of all women. The difference was significant. Our findings are consistent with those described in the doctoral thesis by Hernández-Bel,1 who found that genital warts were more common in women (54.7%).1 Our study provides a more accurate picture of the prevalence of genital warts in our setting, as it includes data on patients seen at the STI unit over a period of 3 years.
Eighty-three percent of the patients in our series were heterosexual, and this predominance is consistent with previous reports from large cohorts in Germany11 and Portugal.10 The mean age of heterosexual patients in our series was 35 years. In the Portuguese series, homosexual patients with genital warts were younger than heterosexuals, but this difference was not detected in our series.
HPV DNA was present in 94% of samples genotyped in our series; this rate is higher than that reported by some authors,15 and slightly lower than that of 99% described by Hernández-Bel.1 Differences may be due to errors in clinical, histopathologic, or molecular diagnoses or to less exhaustive sampling procedures (insufficient tissue, for example), as these were performed as part of routine clinical practice.
We detected 32 distinct genotypes, twice as many as those reported by Rana Al-Awadhi et al.9 in a series of 156 patients in Kuwait. Our findings show a mean of 1.5 genotypes per patient, which is up from 1.1 per patient in the doctoral study published by Hernández-Bel 10 years ago.1 In that study, the maximum number of genotypes per patient was 2 (14% of patients). In our study, 26.6% of patients had 2 or more genotypes, which is very similar to the rate described by Hernández-Suárez et al.5 Coinfections were significantly more common in women, possibly because of systematic vaccination in girls (not boys) or use of different genotyping methods.
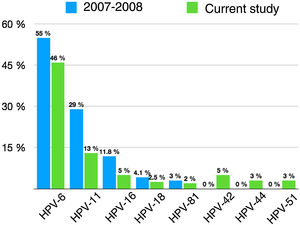
We observed changes in the prevalence of the most common genotypes since prophylactic HPV vaccination was introduced 10 years ago (Fig. 2).
Prevalence of specific HPV genotypes detected in genital warts in the doctoral study by Dr. Hernández-Bel1 (2007–2008) and in the current study.
Twenty-four percent of the patients with HPV DNA in our series had high-risk types, compared with 20% of those in the study by Hernández-Bel.1 The difference is largely due to an increase in genotypes not targeted by the vaccines, such as HPV-51, HPV-31, HPV-52, and HPV-73. None of the high-risk types were significantly associated with any of the clinical or epidemiological variables studied. This is consistent with previous findings,9 although several studies have reported a link between high-risk types and younger age and larger anogenital lesions.9 Just 8 (4%) of the 199 patients had a high-risk genotype without coinfection with a low-risk genotype. Although high-risk types seldom cause genital warts and laboratory errors cannot be ruled out, the rate is similar to rates found elsewhere.1,16
As expected, the prevalence of genotypes targeted by the original bivalent and quadrivalent vaccines (HPV-6, HPV-11, HPV-16, and HPV-18) has decreased. The prevalence of low-risk genotypes in patients without high-risk genotype coinfection, however, has remained stable (75.4% in our series vs. 78.7% in the doctoral study by Hernández-Bel1), largely because of the increase in the number of patients with genital warts caused by HPV-6. This increase could be the result of policy decisions, as, after 2011, the region in which the CHGUV is located administered the bivalent rather than the quadrivalent vaccine, which has proven to be highly effective against the HPV strains most likely to cause genital warts.2
In our series, patients with low-risk genotypes without coinfection with a high-risk type were significantly more likely to be men. Similar findings were observed in 2007–2008 (83.1% for men vs. 75% for women, P=.19).1 Several multicenter studies with larger samples, such as the French study by Aubin et al.,17 also reported significant differences between the presence of high- and low-risk HPV genotypes in men and women.
HPV-6, HPV-11, HPV-16, and HPV-18 were the only genotypes detected in 70.8% of cases in our series; this is lower than the rate of 87% reported 10 years ago1 and can largely be attributed to HPV vaccination in women. Women vaccinated in childhood would have been protected against HPV-6 and HPV-11, and as such, would not have developed lesions had they been infected. Nonetheless, considering that infections with multiple HPV types increased from 14.2%1 to 26.6% after the introduction of HPV vaccines, the presence of these 4 genotypes (alone or in combination) has remained stable (91.4% vs. 92.3%1). This observation puts into context a statement made by Hernández-Bel in his doctoral thesis, that if mass HPV vaccination was achieved in the coming years, the quadrivalent vaccine could prevent approximately 90% of the genital warts analyzed. As our findings show, HPV-6, HPV-11, HPV-16, and HPV-18 are still present in 9 of every 10 genital warts, because vaccination covered girls only. Just 10% of the women in our series had been vaccinated against HPV, a similar proportion to that reported recently in Germany.11 As the mean age of the women was 34 years, most of them were probably too old to have been vaccinated when the vaccines were introduced.
In conclusion, the prevalence of HPV genotypes that have traditionally caused most cases of genital warts and were targeted by the first 2 prophylactic vaccines to appear on the market has decreased in our setting, while other types have become more common. The prevalence of at least 1 of the 4 most common genotypes has remained stable, while that of infections with multiple genotypes or at least 1 high-risk type has increased slightly. The initial policy of using the bivalent rather than the quadrivalent vaccine and to only offer coverage to girls probably contributed to these results. It would be interesting to conduct a large population-based study to determine whether the incidence of genital warts has decreased. This appears to be the case in women who received the quadrivalent vaccine versus the bivalent vaccine or no vaccine at all. Future studies could also assess whether the introduction of the new nonavalent vaccine, which covers high-risk types HPV-16, HPV-18, HPV-31, HPV-33, HPV-45, HPV-52, and HPV-58, and low-risk types HPV-6 and HPV-11, will reduce the presence of high-risk types in genital warts.
Conflicts of interestThe authors declare that they have no conflicts of interest.