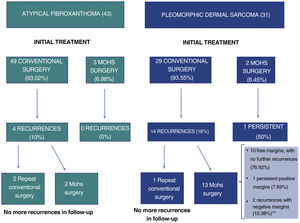
Atypical fibroxanthoma (AFX) and pleomorphic dermal sarcoma are rare mesenchymal tumors.1–4 Historically, the classification of these tumors was based on a changing and imprecise terminology, which has made them difficult to study, and until recently, little data was available regarding their potential aggressiveness.2,5 In 2012, the term pleomorphic dermal sarcoma (PDS) was coined to describe dermal tumors without clear cell differentiation and with histologic characteristics similar to those of AFX, but with clinical and/or histologic characteristic of aggressiveness.1,2,4 The presence of necrosis, lymphovascular or perineural invasion, and/or evident invasion of the subcutaneous cell tissue are the defining histopathology criteria for PDS.1,2,4 Currently, AFX and PDS tend to be considered as part of the same spectrum, with very similar general epidemiologic and histologic characteristics, but with different clinical behavior and aggressiveness.3,6 In this study, we review 43 cases of AFX and 31 cases of PDS diagnosed and treated between 2005 and 2020 at Instituto Valenciano de Oncología and Hospital Clínico Universitario, Valencia, Spain, with the objective of identifying the differences in the clinical and pathologic characteristics and the behavior of the 2 tumors. The clinical and histologic characteristics assessed are shown in the supplemental material (supplemental Tables 1–4), together with the statistical analyses performed. In all cases, the absence of a defined line of cellular differentiation was confirmed, requiring an absence of staining with S100, desmin, pan-cytokeratin (CKAE1-AE3), and CD34. In some cases, additional staining was also performed, including CD10, CD31, and smooth-muscle actin (SMA). Seventy (94.6%) of the cases were men, with a mean age of 79.5 years. A total of 97.3% of the patients had a past history of chronic exposure to sunlight and the most common location of the tumor was the scalp (58.1%). The median size of the lesions was 15mm in diameter (range, 5–60mm). Median time between appearance of the lesion until diagnosis was 3 months (range, 1–48 mo). In terms of the general clinical and epidemiologic characteristics, we found no significant differences between the 2 tumors with regard to distribution by sex, age, location of the tumor, or by time until diagnosis. The median size of the AFX was 13.5mm, whereas that of the PDS was 23mm (P<.001) (Table 1). No significant differences were observed between AFX and PDS with regard to tumor growth pattern, degree of cell pleomorphism, predominant cell type, number of mitoses, ulceration, degree of inflammation, predominant location of the inflammatory infiltrate, and predominant inflammatory cell type. The median tumor thickness of the PDS (7.2mm) was significantly greater than that of the AFX (4.1mm). Furthermore, we found significant differences in the maximum invasion area and presence of necrosis, lymphovascular invasion, perineural invasion, and invasion of the subcutaneous cell tissue (Table 1). In total, 18 cases (24.3%) recurred after the initial treatment, of which 4 (22.2%) were AFX and 14 (77.8%) were PDS. Eight cases (10.8%) were locally advanced at some point in the course of the tumor – all of these were PDS (Fig. 1). The median follow-up time was 16 months (range, 1–228 mo). Four cases (5.4%) presented metastasis (1 lymph node, 2 lung, and 1 lung and brain) and 3 patients (4.1%) died as a result of the disease, all of whom had PDS. Finally, we performed a survival analysis using the Kaplan-Meier estimator and found significant differences between the 2 tumors: disease-free survival after 2 years was 88.2% for AFX, compared to 46.7% for PDS (hazard ratio, 6.1; HR 95% CI, 2.017–18.753; logrank P<.001) (Fig. 2).
Clinical and Pathologic Characteristics That Showed Differences Between Atypical Fibroxanthoma and Pleomorphic Dermal Sarcoma.
| Characteristic | AFX | PDS |
|---|---|---|
| Tumor size, mm | Mean, 14.5Median, 13.5 | Mean, 25.2Median, 23 |
| Tumor thickness, mm | Mean, 4.04Median, 4.1 | Mean, 7.7Median, 7.2 |
| Maximum invasion area | Dermis | 1 dermis17 SCT9 galea aponeurotica3 cartilage1 muscle |
| Necrosis | None | 10/31 |
| Vascular invasion | None | 12/31 |
| Perineural infiltration | None | 10/31 |
| Infiltration of SCT | None | 30/31 |
| Recurrence | None 391 relapse 3More than 1 relapse 1 | None 171 relapse 5More than 1 relapse 9 |
| DFS after 2 y | 88.2% | 46.7% |
Abbreviations: AFX indicates atypical fibroxanthoma; DFS, disease-free survival; PDS, pleomorphic dermal sarcoma; SCT, subcutaneous cell tissue.
Due to the historical confusion that has surrounded the classification of these tumors, the behavior and true potential aggressiveness of AFX and PDS were relatively unknown until recently.2–4,7 Some years ago, the term AFX was used to denote cutaneous tumors, including those with subdermal extension, and several cases of AFX with locoregional and systemic metastasis were described.1–3,7 More recent reviews, however, have clarified the fact that, today, those tumors would be diagnosed as PDS or even as mesenchymal tumors of nondermal origin.3 Since the introduction of the term PDS, the publication of well-defined series of AFX and PDS has allowed for better characterization of the behavior of these tumors. Using strict diagnostic criteria, AFX has eminently benign clinical behavior, whereas PDS may present local aggressiveness in a not insignificant proportion of cases, and may even present distant metastasis.1–3,5,8,9 Nevertheless, the risk factors that make it possible to predict a worse outcome have not been identified and no broad consensus exists to date regarding treatment and follow-up of these tumors.3,7 In the treatment of AFX, Mohs surgery is superior to conventional surgery,10 but sufficient data are not yet available in PDS (although recent studies suggest that Mohs surgery may be more useful).6 In our series, in line with the available literature, we found no differences between the general clinical and pathologic characteristics of the 2 tumors but we did find differences in those characteristics linked to aggressiveness (tumor size, tumor thickness, percentage of recurrences). A considerable percentage of PDS cases recurred after treatment with conventional surgery (Fig. 1) and disease-free survival after 2 years was significantly higher for AFX. In conclusion, we present a broad and well-characterized series of AFX and PDS, which highlight the general clinical and pathologic similarities between the 2 tumors and their different behavior and potential aggressiveness.


![Estimated disease-free survival based on tumor type (atypical fibroxanthoma [AFX] or pleomorphic dermal sarcoma [PDS]): disease-free survival after 2 years was 88.2% for AFX, compared to 46.7% for PDS (hazard ratio, 6.1; logrank P<.001). Estimated disease-free survival based on tumor type (atypical fibroxanthoma [AFX] or pleomorphic dermal sarcoma [PDS]): disease-free survival after 2 years was 88.2% for AFX, compared to 46.7% for PDS (hazard ratio, 6.1; logrank P<.001).](https://static.elsevier.es/multimedia/00017310/0000011300000006/v1_202206190516/S0001731022003465/v1_202206190516/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)




