Cutaneous ultrasound imaging has recently gained ground in dermatology as it has in other specialties (urology, rheumatology, and anesthesiology, for example). Already available to dermatologists in some centers, this tool provides images that can complement the clinical visit, whether for diagnosis or following a patient's response to a course of treatment.1–3
When ultrasound waves emitted by a transducer, or probe, meet a tissue inside the body, an echo is reflected back from the surface of the tissue to the source. At the probe the signal is converted to electrical pulses that are processed to produce an image on a computer screen. The main variables affecting ultrasound images are the frequencies of waves emitted and the depth from which they are reflected back to the probe. In specialties like gynecology or general surgery, low frequencies are used to penetrate deep into the body in order to visualize internal organs. In dermatology, high frequencies (around 15MHz) are used to reach a depth of about 3cm so that superficial structures can be viewed with good resolution. B-mode sonography uses a white-balance method to display images in a range of gray tones between white and black. Various types of Doppler ultrasonography can also be used to complement these B-mode images. In color Doppler mode, ultrasound waves meeting structures that move at a certain velocity, such as blood cells, undergo frequency shifts that are interpreted as red or blue. Pulsed-wave Doppler technology can show whether circulating blood is venous or arterial.
In the hands of an experienced physician, sonography is a rapid, safe, and effective tool that can be an excellent complement to physical examination.
Since June 2013, our dermatology department has been using a high-resolution ultrasound imaging system (SonoScape S20 Exp/S20 Pro/s20/S15) according to an established protocol. Patients are referred for imaging from any of our clinics (general, oncologic, and pediatric dermatology, among others). Candidates might be patients scheduled for surgical treatment or for biopsy of an undiagnosed lesion, children with tumors or vascular lesions requiring evaluation, or patients with an inflammatory or other condition whose disease course or response to treatment must be assessed. The dermatologist assigned to imaging on a given day takes clinical and B-mode or Doppler mode ultrasound images and uploads them to the patient's computerized hospital records. Any dermatologist, whether in a clinic or an operating room, can access the images so the patient's condition can be evaluated fully.
Images might be required in routine pediatric dermatology to assist with the diagnosis of vascular, cystic, or tumoral lesions or to guide the management of treatment. A patient may also be referred for assessment of the presence or absence of inflammatory activity caused by a disease or a foreign body.
Sonography as a complement to the clinical assessment of the vascular lesions of childhood can contribute to the differential diagnosis of infantile hemangiomas and arteriovenous or venous malformations. Hemangiomas appear as more or less well-defined isoechoic lesions in B-mode ultrasound images. Arteriovenous or venous malformations are less well defined and manifest as anechoic tracts with lacunar areas; these areas may have hyperechoic sinus structures (phleboliths in venous malformations). Pulsed Doppler mode can demonstrate whether a hemangioma is in its growth phase (abundant vessels, arterial waveform) or the involuting phase.4 If the target is an arteriovenous malformation, pulsed arterial and venous waves will be seen in combination.
One recent publication provided an example illustrating the utility of cutaneous ultrasound imaging in the diagnosis of complex lesions, specifically the capillary malformation–arteriovenous malformation syndrome linked to the RASA1 gene. It is considered of great utility to observe the presence of 3 or more capillary malformations measuring less than 1–3cm in diameter; these lesions may take either a macular or papular form, be round or oval, and have a whitish halo and/or arterial blood flow confirmed by Doppler ultrasound.5
Sonography can also be of great value in the differential diagnosis of tumors at the lateral corner of the eyelid or in the medial line of the face in children. Images can distinguish between various tumors. A pilomatricoma, for example, is a small tumor with a hypoechoic rim and hyperechoic zones corresponding to calcifications that generate a highly characteristic posterior acoustic shadow. Intact epidermal cysts can appear more or less nodular; they are homogeneous, producing posterior acoustic enhancement and oblique lateral shadows. A channel draining the lesion, or a punctum, may be visible in some images. Lesions that are inflamed are surrounded by increased vascularity. Finally, dermoid cysts are very well defined hypoechoic lesions located in deep layers of the skin. Posterior acoustic enhancement would be unusual. Sonograms of dermoid cysts can provide information about central nervous system involvement or bone remodeling that might require neurosurgical intervention. Once again, it is important to remark that the diagnostic approach must be informed by the clinical signs, so sonography should be used along with any other images that might also contribute to a definitive diagnosis.
Ultrasound imaging is also highly useful for assessing such tumoral conditions as neurofibromatosis or other genodermatoses. Images can be used to identify the borders of lesions and guide surgery. Plexiform neurofibromas, which appear hypoechoic at the periphery (corresponding to a myxomatous region) and hyperechoic at the fibrocollagenous center, can be very complicated to diagnose; multidisciplinary management, involving plastic and/or orthopedic surgery for example, is recommendable.6
Morphea ranks among the most incapacitating inflammatory diseases of childhood because of its rapid progression, possible sequelae, and frequent lack of response to treatment. The usefulness of sonography for evaluating disease activity in morphea was first demonstrated by Wortsman et al.7 and soon confirmed by Lott and Girardi.8 Ultrasound has since been applied to assess response to unconventional uses of drugs such as imiquimod9; this use of ultrasound imaging has not yet been validated in this clinical context, however, so the approach must be considered a complement to clinical evaluation.10
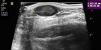
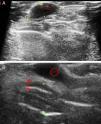
Ultrasound has proven highly useful in adults with a history of cancer. Images can be helpful in alerting the clinician to the development of subcutaneous lesions in patients who have had melanoma or another highly metastatic cutaneous tumor (Merkel cell or squamous cell carcinomas). The presence of well-defined hypoechoic lesions with posterior acoustic enhancement, lateral shadowing, and minimal vascularization (normally just around the lesion) would point to a diagnosis of epidermoid cyst (Fig. 1). If, on the other hand, vessels are found to be abundant inside a similar hypoechoic lesion, the more likely diagnosis will be cutaneous metastasis (Fig. 2). A finding of a well-defined hyperechoic tumor or hyperechoic bands in subcutaneous tissue would suggest a probable diagnosis of lipoma.
A sonogram of a basal cell carcinoma shows hyperechoic dots inside a characteristically hypoechoic lesion. The dots seem to be sonographic representations of anatomic and pathologic structures (calcifications, keratotic cysts, collections of parakeratotic cells, apoptotic cells or necrotic regions in nests of cells) and are fairly indicative of this type of carcinoma, although they have also been described in some melanomas. When Ushara et al.11 studied 29 basal cell carcinomas and 56 melanomas they found 4 hyperechoic dot patterns: A) multiple hyperechoic dots (>5 dots/lesion), B) isolated hyperechoic dots (3–5 dots/lesion), C) multiple moderately echogenic dots, and D) isolated moderately echogenic dots. Pattern A was observed in 48% of the basal cell carcinomas, Pattern B in 24%, pattern C in 10%, and pattern D in 17%. Only 3 melanomas had hyperechoic dots, and all corresponded to pattern D.
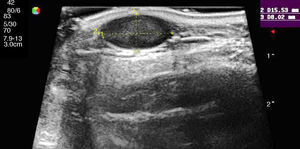
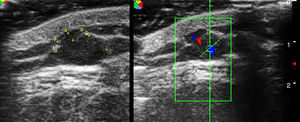
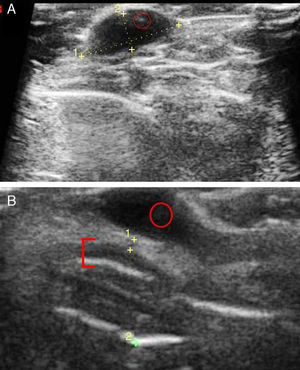
Given that basal cell carcinomas have fairly characteristic sonographic features, images can be of great value in the differential diagnosis of doubtful lesions, for example for distinguishing between pigmented basal cell carcinoma (Fig. 3A) and nodular melanoma. Ultrasound imaging has also been used to establish the perimeter of a lesion, in the sense that it allows the operator to discern whether the carcinoma has invaded nose or ear cartilage (Fig. 3B).
A, Typical ultrasound image of a basal cell carcinoma in the leg: the lesion is hypoechoic, borders are well defined, and hyperechoic dots can be seen inside the carcinoma (faint red circle). B, Basal cell carcinoma after multiple regressions in the left wing of the nose. The basal cell carcinoma (red circle), as described above, can be distinguished from nasal cartilage represented by a hypoechoic band (red bracket).
In benign tumors, such as medial lipomas (forehead, nape of the neck, shoulders), sonography can demonstrate the superficial, intramuscular, or submuscular location of the lesion. Such information can aid in deciding whether or not to schedule surgery with the assistance of an anesthesiologist if the procedure is expected to take long or the patient will require sedation.
After 9 months’ experience with ultrasound imaging, our dermatology department has found this tool to be useful, safe, and rapid. In some cases it can make presurgical biopsies unnecessary. However, diagnostic approaches remain to be validated in many conditions, such as morphea, mentioned above. As dermatologists gain more experience in managing ultrasound imaging devices, their diagnostic utility should increase. In the future, ultrasound imaging will be able to occupy a place alongside thermography and other complementary tools that improve diagnostic precision.
Please cite this article as: Echeverría-García B. Incorporación de la ecografía en Dermatología. Actas Dermosifiliogr. 2014;105:887–890.