Human mpox is a zoonotic infection caused by an orthopoxvirus, with an indolent course that usually resolves within 2–4 weeks. On July 23rd 2022, the World Health Organization (WHO) declared monkeypox virus infections an Emergency of International Concern.1–3 In the current 2022 outbreak, most cases occurred among men who have sex with men and were frequently concomitantly diagnosed with other sexually transmitted infections (STI).3–6
A 58-year-old man with a past medical history of Gaucher disease, Parkinson disease, a kidney transplant on triple immunosuppressive therapy (tacrolimus, mycophenolate, and prednisone), and HIV infection on lamivudine/dolutegravir therapy with undetectable viral load and CD4+ cells>1000/mL. The patient visited the emergency department in July 2022 after 5 days of odynophagia, fever and disseminated mucocutaneus lesions. He reported insertive oral sexual contact 7 days prior to the onset of the symptoms with an unusual partner.
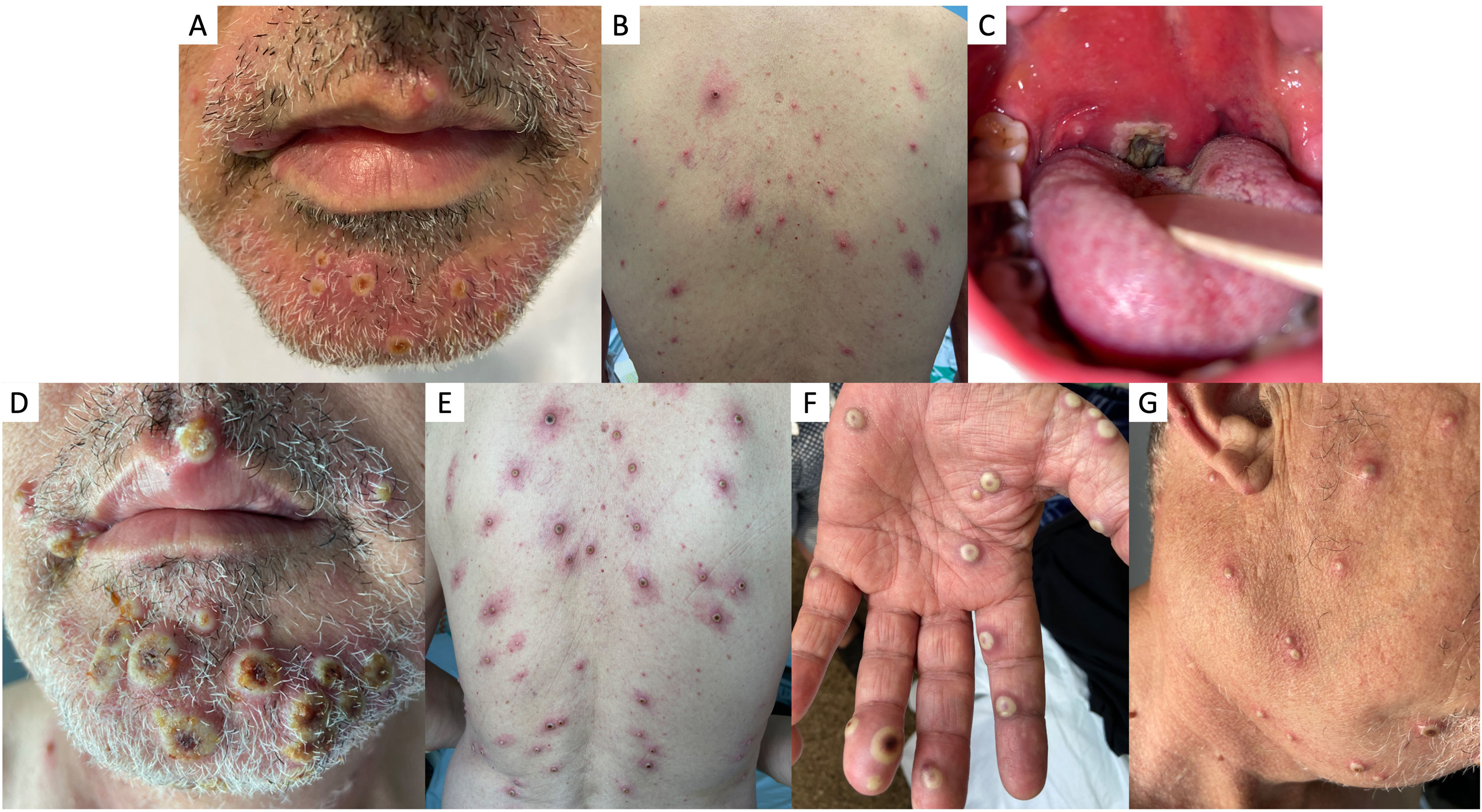
Physical examination revealed the presence of multiple vesicle-pustules on the face, arms, legs, and trunk (Fig. 1A and B), ulcerated lesions in the oral cavity with a necrotic lesion on the right palatine tonsil causing uvular displacement (Fig. 1C), and a large and painful conglomerate of lymph nodes in the right cervical region.
Clinical images of mpox cutaneous lesions: confluent pseudopustules with a depressed necrotic center and perilesional erythema affecting the face (A) and trunk (B) on hospitalization day 1. Ulcerated lesions in the oral cavity and necrotic lesion on the right palatine tonsil with uvular displacement (C). Worsening of the cutaneous lesions on hospitalization day 4: increased number and size of confluent pseudopustules with increased necrotic center on face (E), trunk (F), palms (E), and neck (G).
In the context of the 2022 outbreak, a mpox infection was initially suspected and confirmed as the diagnosis. Pustule-isolated material tested positive for mpox by PCR. Screening for other STIs turned out negative.
The patient had been apparently correctly vaccinated vs smallpox virus in his youth. He was admitted to the Infectious Diseases department, andamoxicillin/clavulanic was initiated. A neck computed tomography revealed the presence of multiple bilateral lymphadenopathies causing a decreased airway caliber.
On day 2, the patient's general condition worsened upon fever presentation, followed by a rapid increase in the number and size of the skin lesions (Fig. 1D–G).
At this point, the administration of antiviral therapy was deemed necessary and a 14-day regimen of 600mg/12h orthopoxvirus Vp37 protein inhibitor tecovirimat was selected and administered orally. Alternative available drugs (cidofovir and brincidofovir) were contraindicated due to the patient's comorbidities. Tecovirimat was individually provided for this patient by the Spanish Agency of Medicines and Medical Devices, given the global outbreak context. As it is a cytochrome inducer, the Pharmacy Department recommended changing the patient's therapy: tacrolimus dose was up-titrated to 4mg/12h with close levels monitoring, mycophenolate was discontinued, and methylprednisolone was up-titrated to 10mg/day as well. The patient showed a slow but favorable clinical course, allowing hospital discharge on day 6 of tecovirimat therapy. Two weeks after therapy completion, basal immunosuppressive therapy was restored.
Most people who contract mpox infection recover within a few weeks without any specific treatment. The most frequent complications are skin bacterial infection and scars.6 Cases of severe complications such as encephalitis, sepsis, pneumonia, airway compression by large cervical lymph nodes, conjunctivitis, keratitis, and myocarditis have also been reported.6,7
About 1–13% of patients require hospitalization8 and the prevalence of fatal disease is between 0 and 11%.2 People at higher risk of severe disease are children, pregnant women, immunosuppressed individuals, older people who have multiple sexual partners, and people from endemic areas.2–4
Following the WHO guidelines, our patient met criteria for severe or complicated mpox infection as he falls into a high-risk population groups due to his immunosuppressive condition. Furthermore, he exhibits clinical signs and symptoms of severity: cervical lymphadenopathy and a severe skin lesion severity score (100–250 lesions). In these cases, the WHO recommends isolation, hospitalization, symptomatic therapy and use of antivirals or other specific therapies, whenever indicated and available.9
The orally bioavailable drugs that have been approved are brincidofovir (FDA) and tecovirimat (EMA). However, efficacy in humans has not been studied yet in randomized clinical trials and its use is poorly reported.10
The smallpox vaccine confers cross protection vs mpox infection with an efficacy of 85%. It was first authorized in July 2022 and is available for pre-exposure prophylaxis of people with high-risk sexual practices, health professionals and post-exposure prophylaxis after high-risk close contacts for severe disease.8 However, there is a lack of data on the efficacy of the vaccine in immunocompromised individuals.4 If smallpox vaccination is contraindicated, a prophylactic IV dose of vaccinia immune globulin is beneficial.4
Mpox has spread rapidly among humans. Although most cases are mild and do not require hospitalization, some patients are at risk of severe disease. More studies are needed to define the risk factors for severity, hospitalization, and mortality to identify those who will benefit from an early antiviral therapy. Currently, several countries have already implemented strategies such as contact tracing, health advice and vaccination campaigns to control the spread of this outbreak.
Conflicts of interestNone declared.