Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe mucocutaneous reactions typically triggered by systemic drugs.1,2 Although topical therapies are also potential inducers of these conditions, their role is poorly defined in literature. The aim of this review is to describe the clinical characteristics of SJS and TEN cases triggered by topical carbonic anhydrase inhibitors (CAIs) reported in the literature, to explore potential underlying mechanisms, and to highlight the limitations of the Algorithm of Drug Causality for Epidermal Necrolysis (ALDEN) in identifying possible triggers for these conditions.
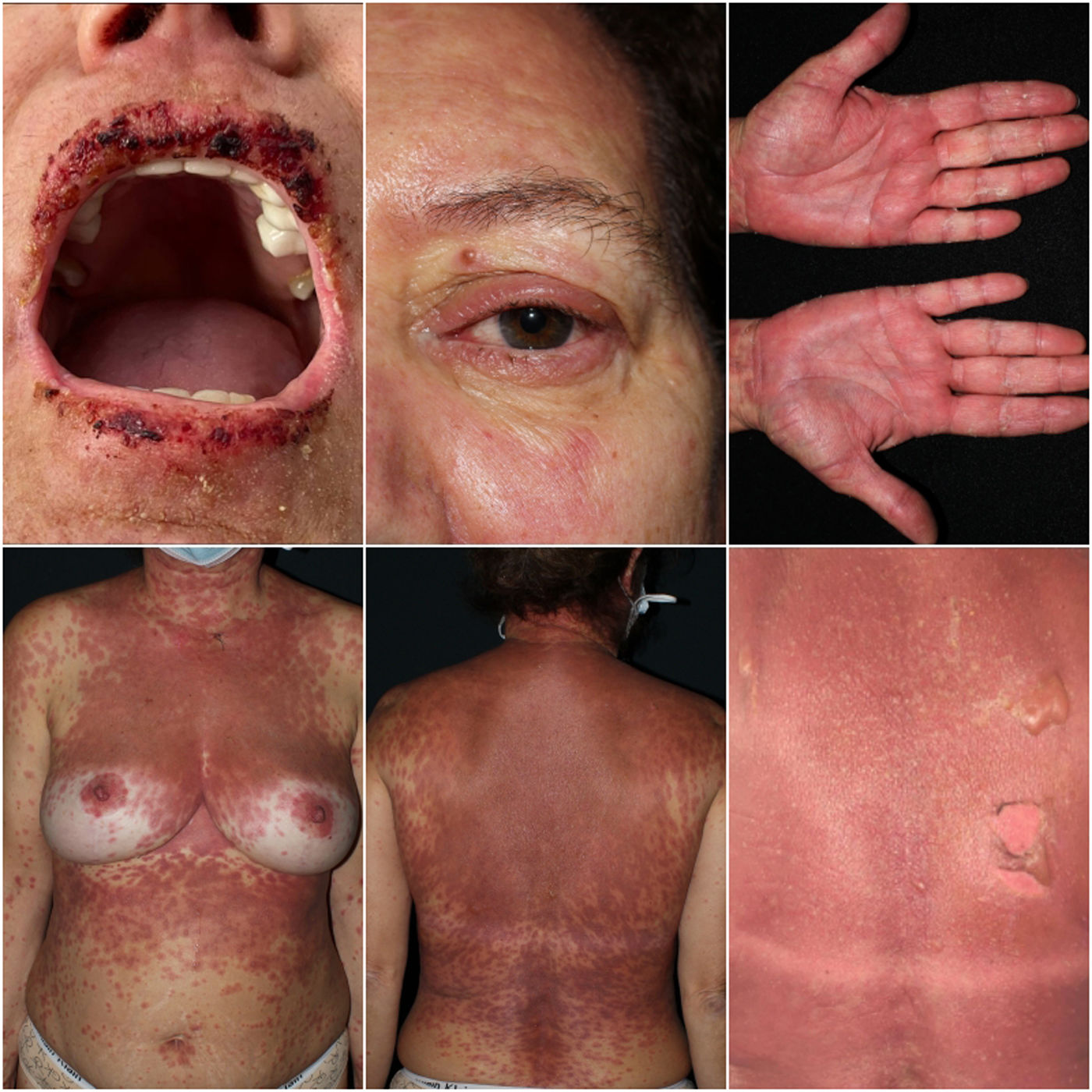
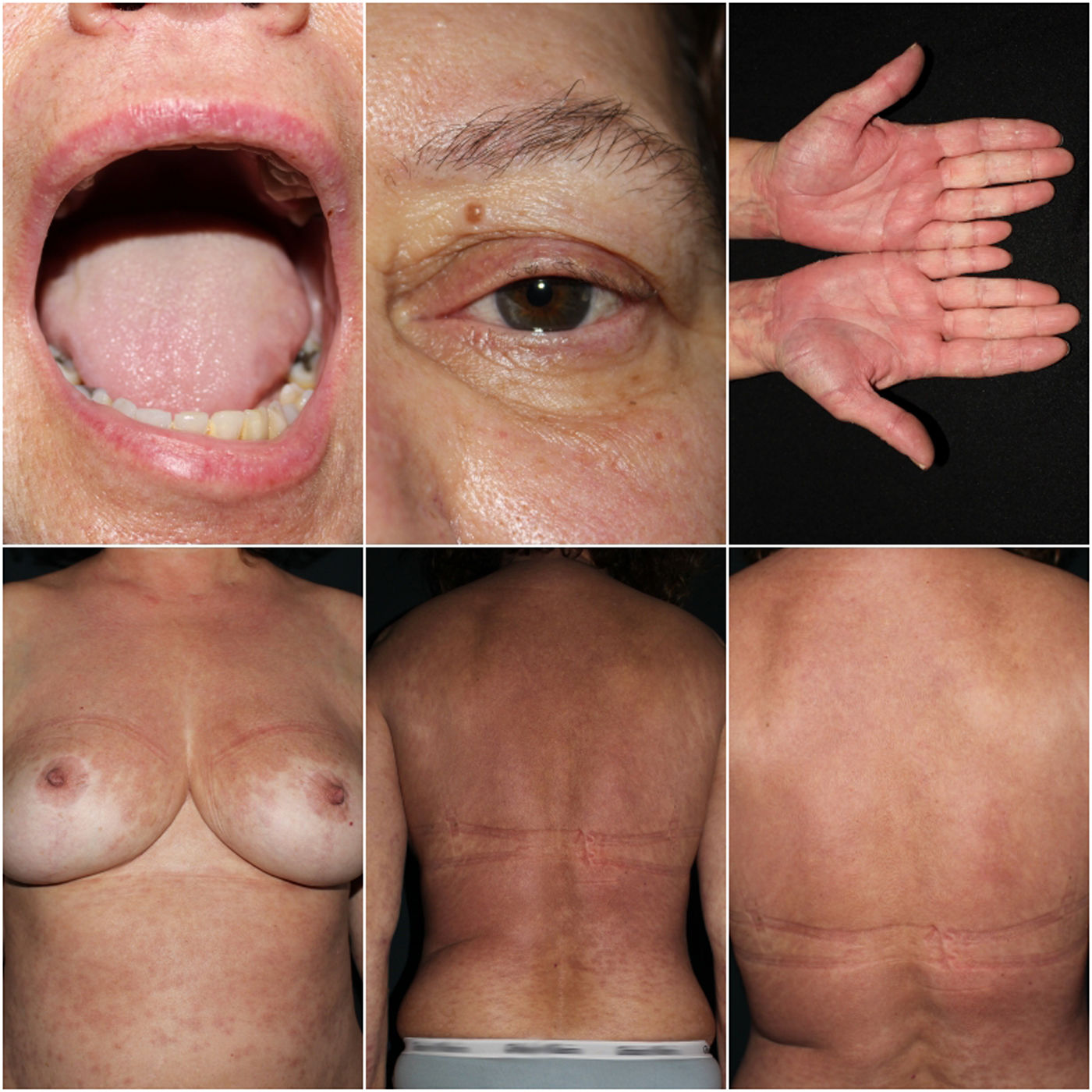
A 57-year-old woman consulted for fever and a painful rash preceded by conjunctival mucositis. The patient's past medical history included hypertension, treated with bisoprolol for 10 years, and chronic glaucoma, for which she had started topical dorzolamide 2 weeks prior to symptom onset. Physical examination revealed the presence of multiple ill-defined, coalescing, erythematous-purpuric macules progressing to blisters and skin detachment over face, trunk and palms (Fig. 1). Skin detachment affected 15% of the body surface area (BSA). She also exhibited oral and conjunctival mucositis. Lab test results demonstrated elevated acute phase reactants and negative serological results, including Mycoplasma Pneumonie. PCR testing for human herpesvirus was performed. Skin biopsy revealed numerous apoptotic keratinocytes with lymphocytic exocytosis and vacuolar interface dermatitis. Based on the above, ALDEN was applied, scoring 3 points, indicating a “possible diagnosis” of SJS/TEN-overlap syndrome due to dorzolamide eye drops. After dorzolamide withdrawal, a single dose of etanercept 50mg was administered along with oral prednisone 30mg per day. In the absence of clinical improvement, oral cyclosporine (100mg every 8h; 5mg/kg/day) and intravenous immunoglobulin (IVIG, 2g/kg administered over 5 days) were added to the treatment regimen. The patient showed a complete resolution of the condition 4 weeks into therapy (Fig. 2).
A literature review of SJS and TEN was performed by searching across PubMed/MEDLINE with the aim of identifying literature cases of SJS and TEN triggered by topical CAIs. The search was updated in January 2024. International articles were included if available in English, Spanish or French. Search terms were “CAI”, “SJS”, “TEN”, “dorzolamide”, “brinzolamide” and “methazolamide”. Articles including topical CAIs were included, but those that involved systemic CAIs were excluded. Those that lacked clinical information about the patients were also excluded. The articles were selected by 2 reviewers based on the predefined criteria.
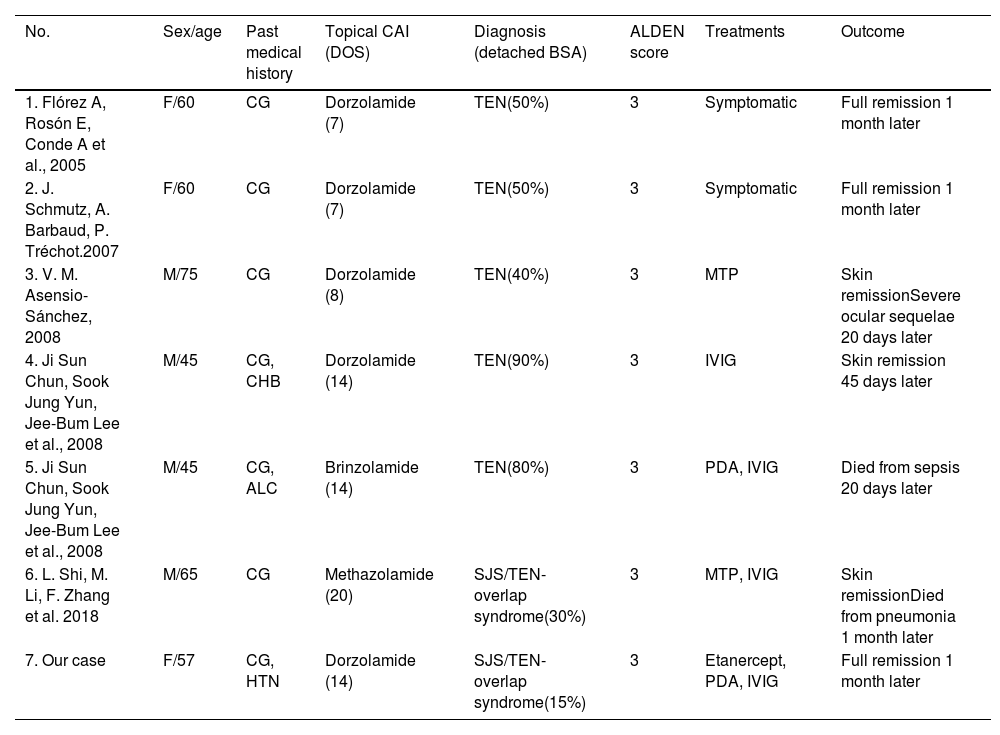
We identified a total of 7 cases associated with the use of topical CAIs (Table 1). The patients were predominantly men (57.1%), with median age 60 years (range, 45–75). Two of them (28.7%) had preexisting liver disease, while the remaining individuals had no other medical conditions or drug intake. ALDEN was applied with a median of 3 points, which indicates a “possible diagnosis”. Four patients (57.1%) exhibited skin detachment covering over 50% of their BSA, with 1 case exceeding 90%. Four of them (57.1%) required IVIG plus corticosteroid therapy, and 2 (28.6%) received symptomatic treatment only. Regarding outcomes, 4 patients achieved full remission without any sequels, 1 (14.2%) developed severe ocular sequelae, and 2 eventually died (28.6%).
Series of Stevens–Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) related to topical carbonic anhydrase inhibitors (CAI) use.
| No. | Sex/age | Past medical history | Topical CAI (DOS) | Diagnosis (detached BSA) | ALDEN score | Treatments | Outcome |
|---|---|---|---|---|---|---|---|
| 1. Flórez A, Rosón E, Conde A et al., 2005 | F/60 | CG | Dorzolamide (7) | TEN(50%) | 3 | Symptomatic | Full remission 1 month later |
| 2. J. Schmutz, A. Barbaud, P. Tréchot.2007 | F/60 | CG | Dorzolamide (7) | TEN(50%) | 3 | Symptomatic | Full remission 1 month later |
| 3. V. M. Asensio-Sánchez, 2008 | M/75 | CG | Dorzolamide (8) | TEN(40%) | 3 | MTP | Skin remissionSevere ocular sequelae 20 days later |
| 4. Ji Sun Chun, Sook Jung Yun, Jee-Bum Lee et al., 2008 | M/45 | CG, CHB | Dorzolamide (14) | TEN(90%) | 3 | IVIG | Skin remission 45 days later |
| 5. Ji Sun Chun, Sook Jung Yun, Jee-Bum Lee et al., 2008 | M/45 | CG, ALC | Brinzolamide (14) | TEN(80%) | 3 | PDA, IVIG | Died from sepsis 20 days later |
| 6. L. Shi, M. Li, F. Zhang et al. 2018 | M/65 | CG | Methazolamide (20) | SJS/TEN-overlap syndrome(30%) | 3 | MTP, IVIG | Skin remissionDied from pneumonia 1 month later |
| 7. Our case | F/57 | CG, HTN | Dorzolamide (14) | SJS/TEN-overlap syndrome(15%) | 3 | Etanercept, PDA, IVIG | Full remission 1 month later |
M: male; F: female; CAI: carbonic anhydrase inhibitor; DOS: days before the onset of first symptoms; BSA: body surface area; CG: chronic glaucoma; HTN: hypertension; ALC: alcoholic liver cirrhosis; CHB: chronic hepatitis B; TEN: toxic epidermal necrolysis; SJS: Stevens–Johnson syndrome; ALDEN: Algorithm of Drug Causality for Epidermal Necrolysis, IVIG: intravenous immunoglobulin; MTP: intravenous methylprednisolone; PDA: oral prednisone; CSA: oral cyclosporine.
SJS and TEN are severe immunomediated reactions characterized by skin detachment and mucosal involvement associated with drug use. The drugs most frequently implicated include anticonvulsants, allopurinol, and sulfonamide antibiotics. In SJS and TEN, the identification of the causative drug is crucial for effective treatment for which the ALDEN is recommended.1–3 However, the algorithm should be applied with caution, as it may underestimate the causality of drugs considered low risk. This limitation arises from the inclusion of the concept of “drug notoriety,” which assigns higher scores to agents with well-established associations, such as sulfonamides.3 CAIs are considered drugs with “unknown” associated risk despite sharing many structural similarities with sulfonamides. For this reason, CAIs may be responsible for systemic reactions despite not scoring high in ALDEN. Therefore, careful interpretation of algorithm results should be exercised.3,4
The use of topical CAIs is widespread for the treatment of ocular hypertension.5 Although SJS and TEN seems to be less frequent with topical than oral CAIs, their topical application is not devoid of risks. Researchers have demonstrated plasma concentrations of CAIs after its topical use,6 which could be explained as topical CAIs are systemically absorbed through the nasopharyngeal mucosa or conjunctival blood vessels.5,6 Therefore, topical CAIs could potentially trigger severe and fatal cases of SJS and TEN in relatively young and healthy patients. Patients with SJS and TEN should be specifically inquired about the use of topical CAIs during the clinical interview as many of them could not consider eye drops as drugs, and they may go unnoticed.
In conclusion, although the use of topical CAIs seems to be safer than their oral administration, they are not devoid of risk. Since topical CAIs are widely used for ocular hypertension, it would be advisable to specifically inquire about these drugs during the clinical interview in patients with SJS and TEN. Although ALDEN is useful, it may underestimate the role of topical CAIs as triggers of SJS and TEN.
Conflict of interestThe authors declare that they have no conflict of interest.
Uncited references7–11.