Alopecia areata (AA) is a dermatological disease of immune origin characterized by partial or total hair loss of the scalp (alopecia totalis) or the whole body (alopecia universalis). Current therapeutic options, both topical and systemic, use nonspecific immunosuppressive or immunomodulatory management strategies. The crucial role of Janus kinases (JAK) in the pathogenesis of the disease has led to a change in the therapeutic landscape, opening new approaches in the management of this disease. Ritlecitinib, a JAK3/TEC kinase inhibitor, has recently been approved for the treatment of severe AA in patients aged 12 and older. This review analyses the mechanism of action of ritlecitinib and the evidence for its safety and efficacy profile in patients with severe AA.
Alopecia areata (AA) is an autoimmune dermatologic disease characterized by partial or complete hair loss of the scalp (alopecia totalis) or the entire body (alopecia universalis), with a variable and unpredictable course. Although its etiology is not fully understood, immunologic, genetic, and environmental factors appear to play a role in its origin.1 AA affects up to 2% of the global population2 and can occur at any age, although in most cases (∼83–88%), it develops before 40 years of age.3 No racial predisposition favoring its occurrence has been identified,4 and both sexes appear to be equally affected,1,4 although some reports suggest a higher incidence rate in women.1,2
The most common symptom of AA is non-scarring hair loss in irregular areas of the scalp. Severe cases may progress to alopecia totalis (AT) or alopecia universalis (AU).5
AA can have a substantial impact on quality of life, including psychosocial burden related to comorbid conditions such as anxiety and depression.2 Additionally, AA is associated with several comorbidities, including thyroid disease, psoriasis, vitamin D deficiency, and other autoimmune disorders.6
The pathogenesis of AA involves loss of immune privilege in the hair follicle (HF) and the subsequent recognition of exposed autoantigens by autoreactive CD8+ T cells through their T-cell receptor (TCR). Interferon-γ (IFN-γ) and interleukin (IL)-15, through a JAK/STAT-mediated signaling response, promote activation and proliferation of autoreactive T cells.7 Moreover, TCR recognition of the autoantigen presented by the major histocompatibility complex (MHC) class I molecule on the epithelial cell of the HF triggers a T-cell signaling cascade involving TEC family kinases, which are crucial for T-cell differentiation and function.8
From a therapeutic perspective, there remains an unmet need for a treatment capable of inducing permanent or long-lasting remission. Given the central role of the JAK/STAT pathway in the pathogenesis of AA, it represents a therapeutic target that has opened new avenues for understanding and managing the disease.5 Currently, two therapies have been approved for severe AA: baricitinib, an oral JAK1/2 inhibitor, approved for the treatment of adults (≥18 years),9 and ritlecitinib, an oral dual selective inhibitor of the JAK3/TEC kinase family, approved for patients aged ≥12 years.
RitlecitinibRitlecitinib 50mg once daily (oral capsules) is the first highly selective covalent inhibitor of the JAK3/TEC kinase family,10 and the only one approved for use in patients aged ≥12 years.
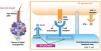
Mechanism of action (MoA)Ritlecitinib selectively inhibits JAK3 kinase as well as TEC family kinases (BTK, BMX, ITK, TXK, and TEC). It binds covalently to a cysteine residue at position 909 (Cys909) in the ATP-binding pocket of JAK3. This Cys909distinguishes JAK3 from JAK1, JAK2, and TYK2, providing a unique mechanism of action (MoA),11 whose dual and selective nature may be advantageous in modulating the pathophysiologic mechanisms involved in AA (Fig. 1).12
Ritlecitinib dual mechanism of action inhibiting JAK3 and TEC family kinases in an AA model.
Created from Divito et al.13.
By inhibiting JAK3, ritlecitinib blocks key cytokines involved in T-cell activation and proliferation, such as IL-15.14In vitro data have demonstrated that ritlecitinib inhibits the cytotoxic function of CD8+ T cells and natural killer (NK) cells, including IFN-γ production, thereby affecting TCR signaling. The reduction of IFN-γ leads to decreased expression of MHC class I molecules15 on the epithelial cell of the HF, reducing recognition of autoantigens by autoreactive T lymphocytes. Moreover, TEC kinases are involved in TCR activation cascades in T cells identified in patients with AA, making their inhibition a relevant therapeutic target in this disease.8
Recently, the MoA of ritlecitinib and baricitinib has been reviewed in the context of AA pathophysiology. While both drugs act on the JAK/STAT signaling pathway, ritlecitinib results in inhibition of a narrower cytokine spectrum due to its selectivity for JAK3, whereas baricitinib inhibits both JAK1 and JAK2. Furthermore, ritlecitinib differs from other JAK inhibitors by also targeting the TEC kinase family. Further research is needed to clarify how these differences translate into clinical safety and efficacy.16
Pharmacokinetics and pharmacodynamicsThe area under the plasma concentration–time curve (AUC) and maximum plasma concentration (Cmax) of ritlecitinib increase approximately proportionally with dose up to 200mg, with a terminal half-life ranging from 1.3 to 2.3h. Peak plasma concentrations occur 1h after multiple oral doses.17
The absolute oral bioavailability of ritlecitinib is approximately 64%, and only 14% of circulating drug binds to plasma proteins. It is metabolized via multiple pathways, including glutathione S-transferase (GST) and cytochrome P450 enzymes. Following a radiolabeled dose, approximately 66% of total radioactivity is recovered in urine (≈4% as unchanged drug) and 20% in feces.17
Ritlecitinib pharmacokinetics are not significantly affected by age (12–73 years), sex, body mass, weight, or race. Food intake has no clinically relevant effect.17
No dose adjustment is required in patients with mild, moderate, or severe renal impairment. However, ritlecitinib has not been studied in patients with end-stage renal disease or renal transplantation, and its use is not recommended in these populations. Similarly, no dose adjustment is needed in mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, though it is contraindicated in severe hepatic impairment (Child-Pugh C). Furthermore, ritlecitinib is contraindicated during pregnancy and lactation, and women of childbearing potential should use effective contraception during therapy and for up to 1 month after discontinuation.17
Clinical evidence for ritlecitinibEfficacy: ALLEGRO phase 2b/3 trialThe phase 2b/3 ALLEGRO study (NCT03732807) was a randomized, double-blind, placebo-controlled trial evaluating ritlecitinib in 718 patients aged ≥12 years with ≥50% scalp hair loss, assessed using the Severity of Alopecia Tool (SALT) – an investigator-rated measure ranging from 0 (no hair loss) to 100 (complete scalp hair loss).8
Patients were randomized to receive 50 or 30mg of ritlecitinib once daily (with or without a 4-week 200mg loading dose regimen), 10mg, or placebo for 24 weeks (Fig. 2). During a subsequent 24-week extension period, the ritlecitinib groups continued with their maintenance doses of 50mg, 30mg, or 10mg once daily, and patients initially assigned to placebo were switched to ritlecitinib 50mg once daily with or without a 4-week 200mg loading dose regimen. The 10mg group was included only in dose–response evaluations.8
Design of the ALLEGRO phase 2b/3 trial. Primary and key secondary endpoints were analyzed at week 24.
Extracted from King et al.8.
The primary endpoint was the percentage of patients achieving a SALT ≤20 at week 24 for the FDA. The primary endpoint for the EMA was the percentage of patients achieving a SALT ≤10 at week 24, and the key secondary endpoint was improvement in the Patient's Global Impression of Change (PGI-C), defined as a rating of “moderately improved” or “much improved” at week 24 (PGI-C: a 7-point patient scale ranging from “very much improved” to “very much worse”).8
Baseline demographic and disease characteristics were balanced among treatment groups: 15% of patients were adolescents, 62% were women, 68% were White, and 46% had AT or AU. The mean baseline SALT score ranged from 88.3 to 93.0, and in patients without AT or AU it ranged from 78.3 to 87.0.8
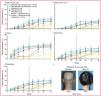
Fig. 3 illustrates the patients who achieved a SALT ≤20, with particular emphasis on the 50mg group, in which 23% achieved this response. The difference in the SALT ≤20 response rate between the placebo and ritlecitinib groups was 21.9% (14.7–30.2; p<0.0001) for this group of patients.8
Patient response based on a SALT score of 20 or less. The difference in SALT ≤20 response rate between the placebo group and the ritlecitinib 200mg+50mg group was 29.1% (95% CI, 21.2–37.9; p<0.0001), 20.8% (13.7–29.2; p<0.0001) for the 200mg+30mg group, 21.9% (14.7–30.2; p<0.0001) for the 50mg group, and 12.8% (6.7–20.4; p=0.0002) for the 30mg group.
Adapted from King et al.8.
A significantly higher proportion of patients achieved a SALT ≤10 at week 24 in the ritlecitinib 30mg and higher groups vs placebo. The proportion of patients with a SALT score ≤20 or ≤10 continued to increase up to week 48 across groups (Fig. 4).8
Patient response by treatment group. (A) Response based on SALT ≤20. (B) Response based on SALT ≤10. (C) PGI-C response (“moderately improved” or “much improved”). (D) Eyebrow response (≥2-grade improvement or a normal eyebrow assessment score of 3 in patients without normal eyebrows at baseline). (E) Eyelash response (≥2-grade improvement or a normal eyelash assessment score of 3 in patients without normal eyelashes at baseline). (F) Representative photos of a responder at week 24. SALT, Severity of Alopecia Tool; PGI-C, Patient's Global Impression of Change. *Statistically significant vs placebo for the overall study at α=0.05.Extracted from King et al.8.
At week 24, between 42% and 52% of patients in the groups treated with 50mg or 30mg of ritlecitinib once daily with or without a 4-week 200mg loading dose regimen had a PGI-C response of “moderately improved” or “much improved” vs 9% in the placebo group. In the 50mg group, the PGI-C response rate was 49%. PGI-C response rates continued to increase beyond week 24 for all 4 groups (Fig. 4).8
All four dose regimens tested met the primary (FDA and EMA) and key secondary (EMA) endpoints at the overall study level and within the hierarchical statistical testing framework agreed upon with these regulatory agencies.8
Furthermore, as in other placebo-controlled AA trials with extensive hair loss (severe),12,14,18,19 the placebo response was very low, confirming the low spontaneous remission rate among patients with ≥50% hair loss.8
Long-term efficacy of ritlecitinibA recent post hoc analysis evaluated individual SALT score trajectories in patients on ritlecitinib 50mg and transitioned from the phase 2b/3 study to the phase 3 ALLEGRO-LT study to describe long-term response patterns and associated baseline disease characteristics. In both studies, patients ≥12 years old with ≥50% scalp hair loss received ritlecitinib 50mg once daily. SALT score trajectories from baseline through month 24 were used to categorize patients as rapid (SALT ≤20 at week 24 and months 12 and 24), intermediate (≤20 at months 12 and 24), late (≤20 at month 24), and partial responders (maintained a 30% improvement), relapsers (achieved but did not maintain a 30% improvement), or nonresponders (did not achieve a 30% improvement).20
The proportions of patients who achieved a sustained response (achieved and maintained SALT ≤20 at all subsequent available time points up to month 24) and a complete response (SALT score 0 at ≥1 time point up to month 24) were assessed. Of the 191 patients on ritlecitinib 50mg, 87 (45.5%) responded (SALT ≤20), 24 (12.6%) partially responded, 24 (12.6%) relapsed, and 56 (29.3%) did not respond. Of the 87 responders, 81 (93.1%) maintained their clinical response and 47 (46.0%) achieved a complete response. Factors associated with treatment response included female sex and lower baseline extent and duration of hair loss. Notably, up to 11% required >1 year of treatment with ritlecitinib to achieve response (late responders), highlighting the importance of extending treatment duration.20
Subgroup analysesThis study was not powered to test hypotheses in subgroups such as adolescents or patients with AT or AU; however, treatment effects in these subgroups were consistent with responses in the overall population across all dose groups.8
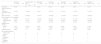
Patients with alopecia totalis or alopecia universalisAmong patients with AT or AU – nearly half of the study population – 7% from the 50mg group achieved a SALT ≤20 at week 24, increasing to 30.9% at week 48 (Table 1).8,21
Proportions of adolescent patients with response based on a SALT score ≤20, SALT ≤10, and PGI-C.
| Ritlecitinib (once daily) | Week 24 | Week 48 |
|---|---|---|
| SALT ≤20 | ||
| 200/50mg | 28% | 25% |
| 200/30mg | 18% | 39% |
| 50mg | 25% | 50% |
| 30mg | 17% | 26% |
| 10mg | 0 | 13% |
| PBO→200/50mg | 0 | 40% |
| PBO→50mg | 0 | 33% |
| SALT ≤10 | ||
| 200/50mg | 28% | 20% |
| 200/30mg | 6% | 28% |
| 50mg | 13% | 33% |
| 30mg | 17% | 26% |
| 10mg | 0 | 13% |
| PBO→200/50mg | 0 | 20% |
| PBO→50mg | 0 | 33% |
| PGI-C | ||
| 200/50mg | 61% | 55% |
| 200/30mg | 53% | 47% |
| 50mg | 59% | 72% |
| 30mg | 45% | 47% |
| 10mg | 22% | 25% |
| PBO→200/50mg | 10 | 80% |
| PBO→50mg | 22 | 44% |
At week 24, patients initially assigned to placebo were switched to ritlecitinib 50mg once daily with or without a 4-week 200mg loading dose regimen. PBO: placebo; 1×/day: once daily; SALT: Severity of Alopecia Tool; PGI-C: Patient's Global Impression of Change. PGI-C response of “moderately improved” or “much improved.”
This group of patients, often refractory to treatment, had higher response rates with ritlecitinib than with placebo, although rates were lower than in patients without AT or AU.8
Patients with abnormal eyebrow or eyelash scoresAmong patients without normal eyebrow or eyelash assessment scores at baseline, improvement by ≥2 grades in eyebrow score or normalization of eyelash score increased over time across all four ritlecitinib groups (Fig. 4).8
Adolescent patientsIn the ALLEGRO-2b/3 trial, 15% of the population consisted of hard-to-treat adolescents with SALT ≥50% (severe AA), a mean disease duration of 6.5 years, and 40% with AT or AU.8 Although limited by small sample size, the results were consistent with those in the overall study population.
At week 24, higher proportions of adolescents from the ritlecitinib treatment groups (≥30mg) had SALT scores ≤20 (17%–28%) and ≤10 (6%–28%) vs placebo and 10mg groups (0%). The proportions of patients with SALT ≤20 or ≤10 at week 48 ranged from 20% to 50% in the ≥30mg ritlecitinib groups (Table 1).8
Among adolescents with AT or AU, 13% (1/8), 25% (2/8), 50% (4/8), and 0% (0/8) achieved SALT ≤20 at week 48 in the ritlecitinib 200/50mg, 200/30mg, 50mg, and 30mg groups, respectively. Moreover, the mean change from baseline in SALT score was greater in the ≥30mg ritlecitinib groups vs placebo through week 24.22
Regarding patient-reported satisfaction and improvement, higher rates were also observed with ritlecitinib vs placebo at week 24. The proportions of adolescents with a PGI-C response of “moderately improved” or “much improved” ranged from 45% to 61% in the ≥30mg ritlecitinib groups at week 24 vs 10% to 22% in placebo groups.22
Pediatric patientsIn pediatric patients, a recent phase 1 study characterized the pharmacokinetic and safety parameters of ritlecitinib in patients with AA aged 6 to <12 years on ritlecitinib 20mg once daily for 7 days.
Fifteen participants were enrolled and 14 (93.3%) completed the study. The median time to maximum plasma concentration (Tmax) for ritlecitinib on day 7 was approximately 0.5h. The half-life of ritlecitinib was approximately 1.19h. The geometric mean (% coefficient of variation) for area under the curve from 0 to 24h (AUC0–24) and maximum concentration (Cmax) were 437.5ngh/mL (30%) and 208.7ng/mL (38%), respectively. Three participants experienced four adverse events (AEs), one of which – urticaria – led to permanent treatment discontinuation. No serious or severe AEs or clinically significant laboratory abnormalities were reported, indicating that ritlecitinib 20mg once daily was generally well tolerated in pediatric patients with AA.23
Impact of prior therapies on ritlecitinib efficacySince patients with AA often receive several therapies over their lifetime, a post hoc analysis investigated associations between prior use of topical AA therapies, intralesional corticosteroids (ILC), topical immunotherapy, systemic immunosuppressants, or any prior AA treatment, and SALT responses in these patients. Of the 522 patients, 360 (69.0%) had prior exposure to any AA treatment. At week 24, a SALT ≤20 response was positively associated with prior ILC use (OR, 2.12; 95% CI, 1.23–3.65; p<0.05) and negatively associated with prior systemic immunosuppressant use (OR, 0.50; 95% CI, 0.28–0.88; p<0.05). Prior use of topical agents or topical immunotherapy was not associated with a SALT ≤20 response at week 24. At week 48, no association was identified between a SALT ≤20 response and prior use of topical agents, ILC, topical immunosuppressants, or systemic immunosuppressants (all p>0.05). Previous exposure to any AA treatment was not associated with a SALT ≤20 response at weeks 24 or 48 (all p>0.05). In conclusion, prior AA treatment history did not influence the long-term response to ritlecitinib.24
Safety of ritlecitinibAllegro phase 2b/3During the placebo-controlled period, AEs that occurred in at least 10% of patients in any treatment group were upper respiratory tract infection, nasopharyngitis, and headache (Table 2).8
Adverse events during the entire study period.
| Placebo to 50mg(n=66) | Placebo to 200mg then 50mg(n=65) | Ritlecitinib 10mg(n=62) | Ritlecitinib 30mg(n=132) | Ritlecitinib 50mg(n=130) | Ritlecitinib 200/30mg(n=129) | Ritlecitinib 200/50mg(n=131) | |
|---|---|---|---|---|---|---|---|
| Permanent discontinuation due to AE | 4 (6%) | 0 | 2 (3%) | 6 (5%) | 4 (3%) | 2 (2%) | 4 (3%) |
| Temporary interruptions due to AE | 8 (12%) | 13 (20%) | 5 (8%) | 16 (12%) | 20 (15%) | 16 (12%) | 17 (13%) |
| Patients with AEs | 57 (86%) | 54 (83%) | 47 (76%) | 106 (80%) | 110 (85%) | 105 (81%) | 108 (82%) |
| AEs occurring in ≥10% of patientse | |||||||
| Headache | 8 (12%) | 8 (12%) | 12 (19%) | 24 (18%) | 16 (12%) | 14 (11%) | 17 (13%) |
| Nasopharyngitis | 4 (6%) | 7 (11%) | 7 (11%) | 21 (16%) | 18 (14%) | 21 (16%) | 19 (15%) |
| Upper respiratory tract infection | 6 (9%) | 7 (11%) | 2 (3%) | 16 (12%) | 11 (8%) | 12 (9%) | 18 (14%) |
| Nausea | 1 (2%) | 8 (12%) | 3 (5%) | 12 (9%) | 3 (2%) | 3 (2%) | 11 (8%) |
| Acne | 8 (12%) | 5 (8%) | 3 (5%) | 12 (9%) | 12 (9%) | 10 (8%) | 6 (5%) |
| Patients with SAE | 3 (5%) | 0 | 2 (3%) | 1 (1%) | 2 (2%) | 2 (2%) | 4 (3%) |
| AEs of special interest, n | |||||||
| Herpes zoster | 0 | 0 | 0 | 0 | 5 | 2 | 1 |
| Serious infections | 0 | 0 | 0 | 1a | 0 | 1b | 2c |
| Pulmonary embolism | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Malignant neoplasms | 0 | 0 | 0 | 0 | 1d | 0 | 1f |
Data express n (%). Summary of AEs, SAEs, discontinuations, and AEs of special interest with ritlecitinib or placebo (safety analysis set). A, adverse event; SAE, serious adverse event.
Table 2 illustrates the common AEs across the entire study (up to week 48, including the follow-up period).8
Integrated safety analysisAccording to an integrated safety analysis25 of data from the ALLEGRO-2a, ALLEGRO-2b/3, and ALLEGRO-LT trials, ritlecitinib was well tolerated in patients with AA. Two cohorts were analyzed: a placebo-controlled cohort from three trials (n=881) and a total-exposure cohort including all patients who received at least 1 dose of ritlecitinib in any of the trials. Pooled data from four clinical trials in the Allegro program with 1294 patients with AA (representing 2092 patient-years of exposure), were consistent with the known safety profile of ritlecitinib.
Ritlecitinib was well tolerated in patients with AA according to an integrated safety analysis including four studies: 3 placebo-controlled (ALLEGRO phase 2a, pivotal ALLEGRO phase 2b/3, ALLEGRO phase 2a safety study) and 1 ongoing long-term open-label phase 3 study (ALLEGRO LT).26
Most AEs were mild, self-limited, and did not require treatment interruption or discontinuation. In the placebo-controlled cohort, AEs occurring in ≥5% of patients included nasopharyngitis, upper respiratory tract infection, and headache. The most frequent AEs (≥2% in any treatment group) that occurred more often in the ritlecitinib groups than placebo and were dose-related included diarrhea, acne, urticaria, rash, and dizziness.21Table 2 lists AEs that occurred in ≥5% of patients in any treatment group within the all-exposure cohort.25
Adverse events of special interestInfectionsThe incidence rate (IR) of serious infections was low across the ALLEGRO program; in this analysis, the IR was 0.66/100 patient-years (PY) in the ritlecitinib 50mg group.21 In a real-world study conducted in the United States, the IR for serious infections was 1.85/100 PY (95% CI, 1.65–2.08) in patients with AA.26
A dose-dependent increase in herpes zoster (HZ) infections was observed with ritlecitinib, which is consistent with former reports for other immunomodulators, including JAK inhibitors (JAKi).27,28 In the 50mg ritlecitinib group from the total-exposure cohort, the IR for HZ was 0.99/100 PY (95% CI, 0.60–1.55); in the real-world cohort with AA from the United States, the IR was 0.78/100 PY (95% CI, 0.65–0.93). Rates were lower in the total-exposure group than in the placebo-controlled group, suggesting no increased risk with longer treatment duration. Additionally, incidence increased with age, and most events resolved within six weeks (median 15 days).26 Moreover, the IR was lower than those reported for HZ in AA patients on baricitinib (JAK1/2) (IR, 1.8/100 PY) and in patients with atopic dermatitis (AD) on upadacitinib (JAK1/2) [IR, 3.8/100 PY (15mg), 6.7/100 PY (30mg)] or abrocitinib (JAK1) [IR, 2.0/100 PY (100mg), 4.3/100 PY (200mg)]. However, further research is warranted to clarify the contributions of the underlying disease vs JAK inhibition to HZ risk.25
Dermatologic eventsThe most frequently reported dermatologic events in the 50mg group and across all ritlecitinib groups included acne (9.0% and 10.4%, respectively), urticaria (6.0% and 6.8%), and folliculitis (5.1% and 6.3%). All were mild to moderate in severity, and few led to treatment interruption or discontinuation.25
MalignancySeven events were categorized as malignant neoplasms (excluding nonmelanoma skin cancer) in the overall exposure cohort [0.32/100 PY (95% CI, 0.14–0.64)]; all were reported in the ritlecitinib 50mg group. Reported malignancies included breast cancer (4), testicular cancer (1), papillary thyroid cancer (1), and melanoma (1), all among the most widely reported cancers in the general population. Five patients had additional risk factors.25
Across the entire ritlecitinib program, 4 cases of breast cancer occurred; the IR in women on any ritlecitinib dose was 0.29/100 PY (95% CI, 0.09–0.70) vs a background IR of 0.46/100 PY (95% CI, 0.33–0.63) in the U.S. cohort study.25
Cardiovascular (CV) eventsFollowing the post-authorization ORAL Surveillance trial of tofacitinib in rheumatoid arthritis,29 it was recognized that JAK inhibitors may increase the risk of thrombosis, major adverse cardiovascular events (MACE), malignancy, and mortality in high-risk populations.30,31 However, it is unclear whether these risks apply to ritlecitinib in AA. Ritlecitinib is a selective dual inhibitor of JAK3 and TEC family kinases, with a distinct mechanism of action. Moreover, patients with AA generally have fewer comorbidities than those with rheumatoid arthritis, and rates of serious infections, HZ, MACE, venous thromboembolism (VTE), and malignancy are lower in dermatologic vs rheumatologic populations.25
Overall, IRs for MACE and VTE in the ALLEGRO clinical program were 0.14/100 PY and 0.06/100 PY, respectively; IRs (95% CI) in the U.S. cohort study were 1.6/100 PY (1.41–1.82) and 0.43/100 PY (0.33–0.54), respectively.26 Notably, patients with CV risk factors were not excluded from the ALLEGRO studies, and all patients with CV events had at least one CV risk factor. More recently, Lo Sicco et al. conducted an integrated CV safety analysis in the 50±200mg ritlecitinib group described above. At baseline, 45% of patients had 0 CV risk factors, 36% had 1, and 19% had ≥2. There were 5 MACE events (5/1228 [0.4%]; IR, 0.21 [95% CI, 0.07–0.48]), 1 VTE (1/1228 [<0.1%]; IR, 0.05 [95% CI, 0.00–0.23]), and 3 arterial thromboembolic events (ATE) (3/1228 [0.2%]; IR, 0.11 [95% CI, 0.02–0.33]). Thus, overall rates of CV and thrombotic events were low in the ritlecitinib clinical program.32
No clinically significant changes in lipid levels were observed during ritlecitinib treatment. Other integrated safety analyses have reported similar IRs of VTE in AA and AD, including baricitinib in AD (IR, 0.09/100 PY)32 and AA (IR, 0.1/100 PY),24 abrocitinib in AD (IR, 0.3/100 PY),33 and upadacitinib in AD (IRs, 0.3/100 PY [15mg], 0.2/100 PY [30mg]).25 EMA safety recommendations for JAK inhibitors (tofacitinib, baricitinib, upadacitinib, abrocitinib, filgotinib) do not include ritlecitinib.33 Additional long-term data are needed to assess VTE risk with selective JAK3/TEC inhibition in AA.25
Neuroaudiological eventsIn chronic preclinical toxicology studies in dogs, reversible axonal dystrophy in the cerebellum was observed with ritlecitinib at exposures ≥7.4 times the approved human dose (50mg).25 At 33 times this dose, axonal dystrophy caused reversible hearing loss and alterations in brainstem auditory evoked potential (BAEP) waveforms. These findings reversed after discontinuation of ritlecitinib. In light of these preclinical results, standard and specialized neurologic and audiologic safety assessments – and adjudication of events – were conducted across the ALLEGRO program.25
Clinical data from the integrated safety analysis, including adjudicated events deemed possible neurologic or audiologic events of interest, showed no evidence of neurotoxicity with ritlecitinib treatment.25
Most AEs associated with neurologic events of interest – such as dysesthesia, hyperesthesia, hypoesthesia, and paresthesia – were mild in severity, considered by investigators to be unrelated to treatment, and resolved spontaneously.25
In a placebo-controlled phase 2a clinical safety study specifically evaluating potential neurologic/neuroaudiologic effects of ritlecitinib in adults with AA using BAEP assessments and intraepidermal axon histology, no notable effects were observed on nerve fiber counts, intraepidermal axon inflammation, or integrity of the human brainstem auditory pathway as assessed by BAEP.25
These data support that the dog finding of axonal dystrophy is not clinically relevant in humans.25
Laboratory assessmentsRitlecitinib was associated with early, dose-dependent decreases in lymphocyte counts; levels recovered toward baseline and remained stable throughout treatment. Early decreases in platelet counts were also observed; these remained stable and above the lower limit of normal throughout treatment.25
ConclusionsRitlecitinib 50mg once daily is the first highly selective, orally administered covalent inhibitor of the JAK3/TEC kinase family and the only agent approved for severe AA in patients ≥12 years of age. Its clinical development has demonstrated superiority over placebo in improvements in the SALT index and other secondary efficacy measures. Across all studies to date, a favorable tolerability and safety profile appropriate for chronic clinical use has been observed.
FundingThis work was funded by Pfizer. The authors received consulting fees from Pfizer S.L.U. for preparation of this manuscript. Medical writing support was provided by Meisys and funded by Pfizer.
Conflicts of interestDr. Sergio Vañó-Galván declared to have received fees as an advisor to Lilly and Pfizer. Dr. Ignasi Figueras Nart declared to have received fees as an advisor and/or speaker for: AbbVie, Celgene, La Roche-Posay, Leo Pharma, Lilly, Novartis, Pierre Fabre, Sobi, Vifor Pharma, Amgen, Pfizer, Galderma, Zuellig Pharma, and Bayer. Dr. Rafael Botella declared to have been an advisor and/or speaker and/or have been involved in clinical trials for: Pfizer, AbbVie, Almirall, Novartis, Janssen, Leo Pharma, Lilly, Celgene, Roche, SunPharma. María del Pilar Fortes and Valeria Herrera-Lasso are employees of Pfizer. All the remaining authors declared no conflicts of interest or personal relationships that could have influenced the work presented in this article.
The authors thank Jorge Fernández-Ortega Martín and Virginia Requena Torres of Meisys for medical writing support.



 Artículo disponible en español
Artículo disponible en español