The relation between atypical fibroxanthoma and pleomorphic dermal sarcoma has led to confusion and debate in the literature. Both tumors present on sun-exposed skin, typically on the head and neck, in patients of advanced age. Both are comprised of a variable mix of histiocytoid, spindle, epithelioid, and/or giant multinucleated cells with pleomorphic nuclei. No immunohistochemical diagnostic techniques have emerged to distinguish these tumors. Diagnosis is by exclusion. Histologically, atypical fibroxanthoma is seen as a well-circumscribed dermal nodule but there will be no evidence of extensive subcutaneous invasion, tumor necrosis, or lymphovascular or perineural invasion. Therefore, if any of the aforementioned features is present, the diagnosis would be pleomorphic dermal sarcoma. This narrative review of the literature aims to identify the distinguishing and overlapping histopathologic features of these 2 tumors as they have been described in case series.
La relación entre el fibroxantoma atípico (FXA) y el sarcoma pleomórfico dérmico (SPD) ha sido confusa y objeto de debate a lo largo de los años en la literatura científica. Son tumores que se presentan en pacientes de edad avanzada en piel fotoexpuesta, típicamente cabeza y el cuello. Están formados por una mezcla variable de células histiocitoides, fusiformes, epitelioides y gigantes multinucleadas con núcleos pleomórficos. No existen técnicas inmunohistoquímicas diagnósticas de estas entidades y su diagnóstico debe ser de exclusión. El FXA es una neoplasia dérmica, bien delimitada, con ausencia de infiltración difusa de tejido subcutáneo, necrosis tumoral o invasión linfovascular o perineural. Estando alguna de las características anteriores presente, debe hacerse el diagnóstico de SPD. En esta revisión narrativa de la literatura intentaremos determinar cuáles son las características histopatológicas precisas de ambas entidades según las series publicadas en la literatura y aquellos aspectos que las diferencian o relacionan.
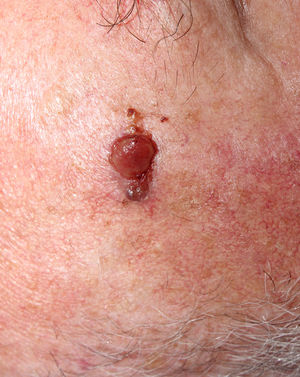
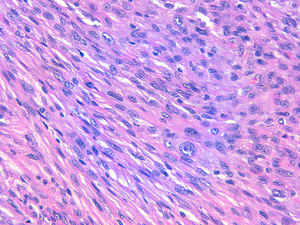
The relation between atypical fibroxanthoma (AFX) and pleomorphic dermal sarcoma (PDS) has led to confusion and debate in the literature over the years.1 Both tumors present in patients of advanced age, predominantly in men, and mainly on sun-exposed skin and on the head and neck, with lesions that are often ulcerated (Fig. 1).2 Both are comprised of a variable admixture of histiocytoid, spindle-shaped, epithelioid, and multinucleated giant cells with pleomorphic nuclei (Fig. 2) often with abundant aberrant mitosis. There are no immunohistochemical diagnostic techniques for these entities and diagnosis should be by ruling out other possibilities.1
These are uncommon tumors and their incidence is unknown, although the incidence of AFX in one Spanish health area has recently been estimated in the literature as 0.59 cases/100 000 inhabitants,3 which contrasts with the incidence of other tumors in Spain, such as basal cell carcinoma (113.05/100 000 inhabitants), squamous cell carcinoma (38.16/100 000), and melanoma (8.76/100 000 inhabitants).4 There are no figures available for the incidence of PDS.
Their etiology, in most cases, is related to chronic exposure to sunlight. The implication of ultraviolet light in their etiopathogenesis is supported by the demonstration of p53 mutations.5
The current histopathologic definition of AFX includes the following characteristics1:
- -
Well-circumscribed dermal neoplasm, comprised of an admixture of histiocytoid, spindle-shaped, epithelioid, and multinucleated giant cells with pleomorphic nuclei
- -
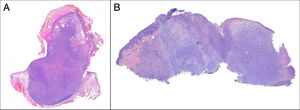
Exophytic, nodular, or polypoid growth (Fig. 3a)
- -
Absence of diffuse infiltrate of subcutaneous cellular tissue, tumor necrosis, or lymphovascular or perineural invasion
- -
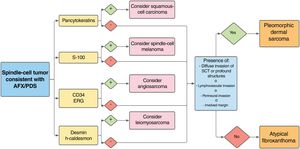
Diagnosis by exclusion based on analysis of the entire resection piece, and after ruling out the main differential diagnoses—squamous-cell, sarcomatous, or spindle-cell carcinoma, spindle-cell melanoma, poorly differentiated leiomyosarcoma, and angiosarcoma—with an appropriate immunohistochemical panel
- -
Possible presence of an epidermal collarette
- -
The deep margin is usually predominantly expansive
PDS has histopathologic features similar to AFX, but with diffuse invasion of subcutaneous cellular tissue or deep structures, tumor necrosis, or lymphovascular or perineural invasion6 (Fig. 3b). When these diagnostic criteria are strictly applied, the clinical behavior of AFX is considered benign, with infrequent local recurrence. Those AFX lesions reported with metastases in the literature are probably other tumors that have been incorrectly diagnosed. The terms undifferentiated pleomorphic sarcoma of the skin and superficial and deep malignant fibrous histiocytoma are poorly documented and not currently used to refer to these tumors.2
Although AFX and PDS are tumors that have been studied in detail, there is still some debate in the scientific literature as to how these entities are related and there are some outstanding questions, such as whether AFX can transform into PDS over time, with the 2 belonging to the same entity and with a prognosis depending on the depth of the neoplasm.1 In this literature review, we will attempt to determine the exact histopathologic features of the 2 entities, according to series published in the literature and those aspects that link them together.
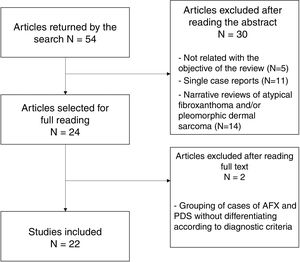
Material and MethodsFor this review, a literature search was performed in the PubMed (Medline), Cochrane Library, Embase, SciELO, and Trip databases with the following terms: “atypical fibroxanthoma” AND “pleomorphic dermal sarcoma,” on September 4, 2020. The initial search retrieved 126 results (53 after eliminating duplicates). The summaries and abstracts were read and screened according to the following criteria:
- -
Criteria for inclusion in the review:
- ◦
Case series published in peer-reviewed journals in the last 10 years, which included AFX and/or PDS, with both tumors described according to currently used terms. Additionally, series that deal with the relationship between these 2 tumors, according to a given feature.
- ◦
- -
Exclusion criteria:
- ◦
Not related with the objective of the review (5 publications)
- ◦
Isolated case reports (11 publications)
- ◦
Narrative reviews of AFX and/or PDS (14 publications)
- ◦
In total, 24 studies were selected for the full text to be read; of these, 22 were finally included in the present review (Fig. 4). Two studies were excluded because of the grouping of AFX and PDS cases, without applying diagnostic criteria for differentiation between them.
Of the series included, the following data were collected: patients and tumor, site, diagnostic criteria used, pattern of invasion, size, mitotic index, ulceration, necrosis, perineural invasion, lymphovascular invasion, involvement of the deep margin, variants, immunohistochemistry (negative markers), prognosis, and relationship between tumors.
ResultsThe results of the series included for AFX,3,7–10 PDS,2,6 and mixed entities11–25 are summarized in Table 1.
Case Series Included in the Review.
| Study | Patients and Tumors | Site | Diagnostic Criteria Used | Invasion Pattern | Tumor Size | Mitotic Index | Presence of Ulceration | TN | Involvement of Deep Margin | Variants/Cytology | Immunohistochemistry (Negative Markers) | Prognosis | Relationship Between Tumors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNI | |||||||||||||
| LVI | |||||||||||||
| Miller et al. (2012)2 | 32 PDS | Head (96.8%) | Invasion of deep SCT (at least deep areas of subcutaneous tissue, muscle, fascia or galea), and/or TN, LVI, PNI | Infiltrative (59.3%) | Median 25mm (range 7 to 60mm) | 19.5 mitosis/10 HGF (range 6 to 51) | 25 (78%) | TN (53%) | 34.3% | Classic 40.6% | AE1/3 | Median follow-up 24 months (available for 29 patients) | NA |
| Forearm (3.2%) | S100, CD34 and desmin negative | Expansive (37.5%) | PNI (28.1%) | Spindle-cell predominance 59.3% | MNF116 | LR 28% | |||||||
| Not determined (3,1%) | LVI (25%) | Myxoid changes 18.7% | cam5.2 | Met 10% | |||||||||
| Pseudoangiomatous/hemorrhagic 9.3% | 34bE12 | ||||||||||||
| Storiform patterns 6.2% | CK14 | ||||||||||||
| Keloid changes 6.2% | S100 | ||||||||||||
| Telangiectatic spaces 3.1% | HMB-45 | ||||||||||||
| Osteoclast-like 3.1% | Desmin | ||||||||||||
| h-caldesmon | |||||||||||||
| CD34 | |||||||||||||
| Thum et al. (2013)11 | 11 AFX | HaN (100%) | Centered on the dermis | Expansive (number of cases NS) | Median 12mm (range 7 to 23) | High (without specifying tumor type) | 10 cases (without specifying tumor type) | Absent | NS (at least 1) | Pseudoangiomatous/hemorrhagic (100%) | AE1/AE3 | Median follow-up 43.1 months (10 patients) | Both tumors negative for ERG and CD34 |
| No evidence of epidermal precursor | MNF116 CK14 CK5/6 S100 melan A | LR 0% | |||||||||||
| HMB-45 Desmin p63 | Met 0% | ||||||||||||
| 3 PDS* | Invasion of underlying fascia | NS | Median 35mm (range 20 to 40) | NS (at least 1) | |||||||||
| Hollmig et al. (2013)26 | 8 PDS | HaN (100%) | Tumor invading SCT | NS | NS | NS | NS | NS | NS (4 cases with biopsy insufficient for assessment) | NS | NS | Median follow-up 19.5 months | Similar immunohistochemical features |
| LR 62.5% | LN-2 is not a good diagnostic or prognostic marker in these tumors | ||||||||||||
| Met 25% | |||||||||||||
| 14 AFX | Tumor limited to the dermis | NS | Mean follow-up 43 months | ||||||||||
| LR 14.2% | |||||||||||||
| Met 14.2% | |||||||||||||
| Zschoche et al. (2014)18 | 25 AFX | HaN (100%) | Pattern of exophytic growth | NS | NS | NS | NS | NS | NS | NS | NS | NS | Both tumors have a similar density of intratumoral lymphatic vessels |
| No invasion of SCT | There are no significant differences between AFX and PDS when the 3 lymphatic subgroups are compared | ||||||||||||
| 22 PDS | HaN (95.5%) | TSC invasion | |||||||||||
| Arm (4.5%) | Tumors in the deep dermis without exophytic growth pattern, still without SCT involvement | ||||||||||||
| Nonaka, Bishop (2014)19 | 19 AFX | HaN (100%) | Tumor limited to the dermis | NS (data grouped by AFX and PDS) | NS (data grouped by AFX and PDS) | NS (data grouped by AFX and PDS) | NS (data grouped by AFX and PDS) | NS (data grouped by AFX and PDS) | NS | NS | AE1/AE3 | NS (data grouped by AFX and PDS) | Not evaluated because pooled data are presented |
| MNF116 | |||||||||||||
| 34bE12 | |||||||||||||
| CK5/6 | |||||||||||||
| CK14 | |||||||||||||
| p63 | |||||||||||||
| 34 PDS | Tumor extends to the SCT | ||||||||||||
| Griewank et al. (2014)20 | 27 AFX | HaN (96.2%) | Absence of invasion of SCT, TN, PNI, and LVI | Expansive (100%) | Median 9mm (range 4-30) | Median 20 mitosis/mm2 (range 7-53) | 44.4% | Absent | NS | NS | MNF116 | NS | Mutations in the TERT promotor are present in 93% of AFX and 76% of PDS. The number of CC>T mutations suggests a pathogenic role for UV light in both tumors |
| Unknown (3.8%) | AE1/ AE3 | ||||||||||||
| CD31 (focal in 1 case) | |||||||||||||
| CD34 | |||||||||||||
| S100 | |||||||||||||
| Desmin | |||||||||||||
| 34 PDS | HaN (94.2%) | Presence of invasion of SCT, TN, PNI, or LVI | Invasive (50%) | Median 22mm (range 6-60) | Median 19 mitosis/mm2 (range 5-51) | 76.4% | TN (55.8%) | ||||||
| Unknown (5.8%) | Expansive (47%) | PNI (26.4%) | |||||||||||
| Not evaluable (2.9%) | LVI (26.4%) | ||||||||||||
| Harding-Jackson et al. (2015)7 | 15 AFX | HaN (86.6%) | Circumscribed with expansive border | Expansive (100%) | NS | Range 5-38 per 10 HGF | 60% | Absent | NS | NS | Desmin Calponin h-caldesmon | At least 2 years of follow-up | NA |
| Unknown (13.3%) | Absence of TN, PNI, LVI or deep extension to SCT | S100 | LR 6.6% (1 case) | ||||||||||
| P63 | Met 0% | ||||||||||||
| Broad spectrum CK | |||||||||||||
| CD31 | |||||||||||||
| CD34 | |||||||||||||
| Wang et al. (2015)8 | 11 AFX | HaN (100%) | Include tumors with SCT, TN, and PNI invasion | Invasive (90.9%) | Median 8mm (range 3-18) | Median 20 mitosis per 10 HGF (range 10-55) | 27.27% | TN 18.18% | NS | Spindle-cell (6/11) | NS | Selection of 9 cases with metastasis in 152 (5.9%) + 2 selected from another center | NA |
| Not available (9.1%) | PNI 45.45% | Spindle-cell with isolated giant cells (2/11) | 2 cases without SCT involvement metastasized | ||||||||||
| No LVI | Spindle-cell and epithelioid (1/11) | ||||||||||||
| Spindle-cell and pleomorphic (1/11) | |||||||||||||
| Pleomorphic (1/11) | |||||||||||||
| Tardío et al. (2016)21 | 18 PDS | HaN (100%) | Invasion of deep subcutaneous tissue and/or TN and/or PNI and/or LVI | Expansive (17%) | Median 15mm (range 7-70) | Median of 22 mitosis per 5 mm2 (range 2-126) | 55.5% | TN (17%) | 22.2% | Spindle-cell fascicular | Cytokeratins | Median follow-up of 33 months (available in 15/18) | Same histopathologic and immunohistochemical features, except for lack of invasion of deep SCT, TN, or LVI in AFX |
| Invasive (83%) | LVI (17%) | Giant multinucleated epithelioid cells (2/18) | S100 | Only differences in prognosis | |||||||||
| Neoplastic cells with moderately pleomorphic vesicular nuclei and prominent nucleoli | Desmin | LR 20% | |||||||||||
| CD34 | Met 20% | ||||||||||||
| 45 AFX with follow-up of more than 12 months (for comparison) | HaN (100%) | Absence of deep subcutaneous tissue invasion, TN, PNI, or LVI | NS | Median 11mm (range 4-30) | NS | NS | Absent | NS | NS | NS (the same) | Follow-up of more than 12 months (median 48 months)LR 2.2% (1 case) No Met | ||
| Helbig et al. (2016)22 | 5 AFX | Thigh (20%) | Limited to the dermis | NS | NS | NS | NS | NS | NS | NS | At least 1 cytokeratin (CK 5/6 or pancytokeratin) | NS | All AFX and PDS presented similar oncogene expression (overexpression of TP53, CCND1, CDK4) |
| HaN (80%) | Two melanocytic markers (S100, melan A, or HMB-45) | One case with AFX and PDS showed mutational profiles of TP53 and PIK3CA, identical in both tumors | |||||||||||
| Vascular markers (CD31, CD34, or podoplanin) | |||||||||||||
| 6 PDS (1 patient with AFX and PDS separated by 3 years) | HaN (100%) | TSC invasion | |||||||||||
| Helbig et al. (2017)23 | 5 AFX | HaN (80%) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | Amplifications and deletions detected in 6 of 27 PDS; these were not detected in AFX |
| Unknown (20%) | |||||||||||||
| 26 PDS | HaN (96.1%) | ||||||||||||
| Shoulder (3.9%) | |||||||||||||
| Griewank et al. (2018)24 | 41 AFX | HaN (90.2%) | Established after analysis | Invasive (2.4%) | Median 8mm (range 4-30) | Median of 17 mitosis/mm2 (3-52) | 34.1% | TN 2.4% (1 case included) | NS | NS | Pancytokeratin (MNF116, AE1/AE3) CD31, or CD34 | NS | AFX and PDS present recurrent mutations in FAT1, NOTCH1/2, CDKN2A, TP53, and the TERT promotor |
| Arm (4.8%) | SS association of PDS with TN, SCT invasion, invasion of fascia/muscle, involved border, and LVI | Expansive (73.1%) | Not determined 17.1% | No PNI | S100 | ||||||||
| Unknown (4.8%) | Not evaluable (24.3%) | No LVI | Desmin Smooth muscle actin melan A (1 case of aberrant expression) | ||||||||||
| 40 SPD** | HaN (92.5%) | Invasive (40%) | Median 20 (range 4-60) | Median of 21 mitosis/mm2 (range 5-44) | 55% | TN 40% | |||||||
| Arm (2.5%) | Expansive (25%) | Not determined 25% | PNI 22.5% | ||||||||||
| Unknown (5%) | Not evaluable (35%) | LVI 20% | |||||||||||
| Helbig et al. (2018)25 | 25 AFX | HaN (96%) | No invasion of SCT | NS | NS | NS | NS | NS | NS | NS | AE1/AE3 | NS (only available for the 2 patients with MiTF expression: free of tumor progression) | Expression of MiTF in 1/25 AFX and 1/25 PDS; expression of α-SMA in 40% of AFX and 36% of PDS |
| Leg (4%) | CK5/6 | ||||||||||||
| p40 | |||||||||||||
| SOX10 | |||||||||||||
| ERG | |||||||||||||
| CD10+ necessary | |||||||||||||
| 25 PDS*** | HaN (92%) | Invasion of SCT, fascia, or muscle | |||||||||||
| Shoulder (8%) | |||||||||||||
| Gaiser et al. (2018)13 | 51 AFX | HaN (100%) | NS | NS | NS | NS | NS | NS | NS | NS | NS | LR 1.9% (1 case) | MYC amplification is not a determining genetic factor in the process of tumor genesis for AFX or PDS |
| No Met | |||||||||||||
| 24 PDS | HaN (91.7%) Shoulder (8.3%) | NS | |||||||||||
| Koelsche et al. (2019)14 | 17 AFX | HaN (100%) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | The DNA methylation profile does not distinguish between AFX and PDS |
| Loss of 9p and 13q and gain of 8q with similar frequency | |||||||||||||
| Homozygous deletion of CDKN2A more frequent in PDS than AFX | |||||||||||||
| 15 PDS | HaN (93.3%) | ||||||||||||
| Unknown (6.6%) | |||||||||||||
| Müller et al. (2019)9 | 41 AFX (40 patients) | HaN (90.3%) | NS | NS | Mean of 5.7 cm2 (range 0.06 and 40) | Median 4.8 mitosis/10 HGF (range 1-50.4) | 68.2% | TN 14.6% | NS | NS | NS | LR 7.3% | NA |
| Limbs (9.7%) | Includes patients with Clark level V and necrosis but without LVI | No PNI | No Met | High expression of SEC62 in tumors with necrosis, size > 5 cm2, high Clark levels | |||||||||
| No LVI | |||||||||||||
| Nassios et al. (2019)15 | 4 AFX | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | Similar expression of GPR4, TDAG8, OGR1, and G2A in AFX and SPD studied |
| 5 PDS | |||||||||||||
| Miller et al. (2020)16 | 21 AFX | HaN 90.5% | Atypical spindle-cell proliferation in sun-damaged skin with minimal or no SCT invasion, without TN, LVI, PNI, or epithelial/melanocytic/vascular/smooth muscle differentiation by morphology or immunostaining | NS | Median 0.9mm (range 0.2-2.8) | NS | NS | Absent | NS | NS | Cytokeratins | Follow-up in 7 patients | Mutations detected in PIK3CA (and not in PDS), more frequent CDKN2A deletions in PDS compared with AFX, CDKN2a mutations in AFX were not observed in PDS but were observed in AFX, increased mutations in the TERT promotor in PDS compared with AFX. |
| Knee 4.7% | S100 | No LR nor Met | |||||||||||
| Chest 4.7% | Others (see supplementary table in original article) | ||||||||||||
| 17 PDS | HaN 88.2% | Presence of deep subcutaneous invasion, necrosis, lymphovascular invasion, and/or perineural invasion | NS | NS | NS | NS | NS | NS | Follow-up in 2 patients (both LR) | ||||
| Thigh 5.8% | |||||||||||||
| Supraclavicular 5.8% | |||||||||||||
| Lonie et al. (2020)6 | 27 PDS | HaN (96.3%) | Cytokeratins, S100, and CD34 negative, without specifying other histopathologic features | NS | NS | Numerous 35% | NS | NS | NS | NS | Cytokeratins | Median follow-up of 46.4 months | NA |
| Trunk (3.7%) | Frequent 33.3% | S100 | |||||||||||
| CD34 | LR 7.3% | ||||||||||||
| Met 3.7% (1 case) | |||||||||||||
| Ricci et al. (2020)17 | 5 AFX | HaN (100%) | NS | NS | NS | NS | NS | NS | NS | NS | Cytokeratins | NS | Cathepsin-K was moderately positive and diffuse in all cases of AFX and PDS |
| 4 PDS | HaN (50%) | ||||||||||||
| Trunk (50%) | |||||||||||||
| Bitel et al. (2020)10 | 105 AFX | HaN (97.9%) | NS | NS | 15mm +/- 3mm | High 63% | 47.6% | NS | NS | Spindle-cell 70% | NS | Mean follow-up of 30 months in 36 patients | NA |
| (85 patients) | Shoulder (2.1%) | Includes tumors invading muscle/fascia/cartilage | Intermediate 17.8% | Mixed 30% | LR 22.9% | ||||||||
| Low 19.2% | No Met | Identification as a risk factor for recurrence of invasion of deep structures | |||||||||||
| Iglesias-Pena et al. (2020)3 | 62 AFX | HaN (96.8%) | Invasion of deep structures | NS | Mean 12.3mm (range 3-40) | Median of 7.09 mitosis/10 HGF (range 0-31) | 50% | Absent | 8.1% | Classic 88.7% | AE1-AE3 | Median follow-up of 34 months | NA |
| Trunk (3.2%) | Compatible immunohistochemical panel | Nonpleomorphic spindle-cell 4.8% | CD34 | LR6,5% (4 cases) | |||||||||
| Absence of TN, PNI, and LVI | Hemosiderotic 4.8% | HMB45 | No Met | ||||||||||
| Keloid 4.8% | Desmin | ||||||||||||
| EMA | |||||||||||||
| S100 (1 focal) |
Abbreviations: AFX, atypical fibroxanthoma; HaN, head and neck; HPF, high power field; LR, local recurrence; LVI, lymphovascular invasion; Met, metastasis; NA, not applicable; NS, not specified; PDS, pleomorphic dermal sarcoma; PNI, perineural invasion; SCT, subcutaneous cellular tissue; SS, statistically significant; TN, tumor necrosis.
*Included in the series of Miller et al. (2012).
**20 of these included in the series of Miller et al. (2012).
***Four of these included in the series of the same author Helbig et al. (2016).
The series are in general small, with few patients, rarely exceeding 30 tumors for each type. The site was mainly the head and neck, with all tumors reported at this site in many of the series.
Differences Between AFX and PDSAFX and PDS were differentiated in most of the series with histopathologic criteria. In the series reported by Griewank et al.24 in 2014, differentiation between AFX and PDS was performed by 2 dermatopathologists and in a subsequent analysis it was found that necrosis, invasion of subcutaneous tissue, invasion of fascia/muscle, involved margin, and lymphovascular invasion are criteria significantly associated with PDS.
The diagnostic criteria used to differentiate between AFX and PDS are not homogeneous in the studies, and often they are not clearly specified. The tumors with tumor necrosis, lymphovascular invasion, perineural invasion, or invasion of the fascia/muscle were generally classified as PDS, although some series classified tumors with some of these features as AFX.8–10,24
Invasion of subcutaneous cellular tissue deserves special mention for differentiating between AFX and PDS. In some series,12,18–20,22,25 simple invasion of subcutaneous cellular tissue is automatically considered a criterion for PDS whereas in others,2,7,11,21 this infiltration should be deep, in some cases without specifying how deep. One series went as far as to consider PDS those dermal tumors that did not invade subcutaneous tissue because they did not have a exophytic pattern of growth (Table 2).18
Differences in Considering AFX and PDS, According to Subcutaneous Tissue Invasion in Some of the Case Series Included.
| Simple Invasion of SCT as Criterion for PDS | Deep Invasion of SCT Required to Consider PDS Diagnosis |
|---|---|
| Hollmig et al. (2013)26 | Miller et al. (2012)2 |
| Zschoche et al. (2014)18 * | Thum et al. (2013)11 |
| Nonaka, Bishop (2014)19 | Harding-Jackson et al. (2015)7 |
| Griewank et al. (2014)20 | Tardío et al. (2016)21 |
| Helbig et al. (2016)22 | |
| Helbig et al. (2018)25 |
*PDS was considered even without SCT invasion if tumor growth was not exophytic.
Abbreviations: PDS, pleomorphic dermal sarcoma; SCT, subcutaneous cellular tissue.
Many series did not specify the pattern of invasion of the tumors. When this was specified, most AFX had an expansive pattern, whereas the patterns reported for PDS had a mainly infiltrative pattern of growth.2,7,20,21,24 Of note is the percentage of AFX with invasive pattern in the series of Wang (90.9%), with 11 metastatic AFX lesions, which included tumors with extensive invasion of subcutaneous tissue, tumor necrosis, and perineural infiltration; these are criteria that should trigger reclassification as PDS.8
Tumor size11,20,24 and mitotic index,20,24 when reported, are clearly larger in PDS than AFX. Likewise, ulceration is reported more often in PDS in studies in which this feature is reported separately.20,24
In the series of AFX by Müller et al.,9 a significantly larger increase in SEC62 expression is reported in tumors with necrosis and a trend to greater expression in tumors with high Clark levels and tumor size greater than 5cm2.
At the genetic level, of note is the series published in 2020 by Miller et al.,16 who reported mutations in PIK3CA (and not in PDS), more frequent CDKN2A deletions in PDS compared with AFX, CDKN2a mutations in AFX that were not observed in PDS, and increased mutations in the TERT promotor in PDS compared with AFX.
Finally, in terms of prognosis, differences between AFX and PDS are noteworthy. In series that included both types of tumor,21,26 local recurrence and metastases occur markedly more frequently in PDS. Of note is that the series that report metastatic AFX with specification of histopathologic features8,10 include tumors with invasion of deep structures, tumor necrosis, or perineural invasion (which, according to the current criteria, would be considered PDS).
Similarities between AFX and PDSIn this review, we can identify similarities between AFX and PDS in the different series that compare these tumors.
In the immunohistochemical studies, no study identified a marker that was expressed differently in the 2 types of tumor.26 The immunohistochemical markers used vary from one study to another, but most include some cytokeratins, S100, and vascular markers (CD31, CD34), although this is not always clearly specified.8–10,12–15,18,23
The investigations by Zschoche et al.18 did not show any differences in lymphatic architecture in more than 20 examples of each type of tumor.
In the series of Nonaka and Bishop,19 AFX and PDS are presented grouped as sarcoma-like tumors, with no immunohistochemical evidence of epithelial differentiation and no histologic signs of a squamous-cell carcinoma component. This meant that the characteristics of these tumors could not be assessed separately for the purposes of this review. Interestingly, these authors showed that the prognosis for AFX and PDS, when these tumors are grouped together, was similar to that of other sarcoma-like tumors with an epithelial component, suggesting that at least some cases of AFX and/or PDS could be related to squamous-cell carcinoma, with the former representing complete loss of epithelial phenotype.
At the genetic level, the series by Griewank et al.20 published in 2018 shows that mutations in the TERT promotor are present in 93% of AFX and in 76% of PDS. The number of CC>TT mutations suggests a pathogenic role for UV light in both tumors. In the series reported by Helbig et al.,22 AFX and PDS were found to have a similar expression of oncogenes, with overexpression of TP53, CCND1, and CDK4, leading the authors to reaffirm their hypothesis that AFX is the noninvasive precursor of PDS. In another series reported by Helbig et al.25 in 2018, with 25 AFX and 25 PDS lesions, the authors found MiTF expression in one AFX and one PDS, as well as expression of α-SMA in 40% of AFX and 36% of PDS. Gaiser et al.13 also ruled out amplification of the MYC gene as an important process in tumor genesis of AFX and PDS. Koelsche et al.14 demonstrated that the DNA methylation profile does not distinguish between AFX and PDS; in their study they observed loss of 9q and 13q and gain of 8q in a similar frequency in both tumors. Homozygous CDKN2A deletion was most frequent in PDS (6/15) compared with AFX (2/17), although the sample size was relatively small.
Nassios et al.15 studied expression of proton-sensitive protein G coupled receptors and found similar expression of GPR4, TDQAG8, OGR1, and G2A in the AFX and PDS studied.
DiscussionExcept for the aforementioned histopathological criteria (invasion of deep structures, tumor necrosis, perineural invasion, and lymphovascular invasion), it is not clear where the differences between AFX and PDS actually lie. The scientific literature is very confusing due to the different terminology, different diagnostic criteria used by the authors, and insufficient immunohistochemical characterization of the tumors,27 especially in older articles.28 It is essential to perform an exhaustive assessment of resected pieces when such tumors are suspected, as the final diagnosis has implications in the subsequent management of the patients.29
Variants of AFX have been described in the literature and knowledge of these is important to avoid diagnostic errors. These include nonpleomorphic spindle-cell AFX, clear-cell AFX, hemosiderotic AFX, myxoid AFX, AFX rich in osteoclast-like giant cells, keloid AFX, and granular cell AFX.1 These changes have also been reported in some PDS, either involving the tumor areas or the entire lesion.2
In AFX, a chronic inflammatory infiltrate can be observed, above all in the tumor periphery and the presence of severe solar elastosis is usually observed in the adjacent dermis.5
The immunohistochemical markers that can help differentiate between AFX and other tumors include CD99, S-100, CD34, cytokeratins, desmin, CD10, vimentin, HMB-45, CD68, and p63, among others.9 Differential diagnosis of these tumors with angiosarcoma is important and CD34 and ERG have been shown to be useful in this context, particularly in AFX-PDS with pseudoangiomatous/hemorrhagic pattern (Fig. 5).11 To date, attempts to differentiate between AFX and PDS with immunohistochemical markers (CD99, LN-2) have not proved successful.12,30
Strong positivity for CD10 in AFX and PDS can support diagnosis. However, it is important to note that any tumor with spindle-cell morphology can be positive for this marker, including sarcomas such as mixofibrosarcoma.5
It is also important to highlight that dendritic cells present in biopsies of AFX and PDS are positive for S100, but the tumor cells should be negative for this marker to make a diagnosis.5
Of note in this sense is the series of by Müller et al.,9 in which a significant increase in SEC62 expression was reported in tumors with necrosis and a trend to greater expression in tumors with high Clark levels and tumor size greater than 5 cm2. Although the histopathologic features for diagnosis of AFX are not specified in the study, the fact that large tumors with necrosis and subcutaneous invasion were included suggests that, according to current criteria, some tumors would actually be PDS. This increased expression of SEC62 in PDS compared with AFX could be the object of new lines of research in the future, with inclusion of both types of tumor with stricter diagnostic criteria.
Given that diagnosis of AFX and PDS is by exclusion, negative immunohistochemical markers suggestive of other entities are those that are used for ruling them out. In the literature, the number and type of markers that need to be negative for diagnosis have not been established. Thus, in many cases series, immunohistochemical characterization of tumors is insufficient and can, in actual fact, correspond to other entities.31
It is worth asking whether, faced with tumors as closely related as AFX and PDS, it makes sense to continue using different terms. In situ melanoma does not have the same prognosis as ulcerated melanoma, with lymphovascular invasion and microsatellitosis, but we still refer to both lesions as melanoma. If to date, no significant differences have been found at the cellular, immunohistochemical, genetic, or molecular level, it makes sense to group these tumors in the same spectrum and use the same term to describe them. This is what Winchester et al.32 did in a series of 319 patients with tumors encompassed by the term undifferentiated pleomorphic sarcoma, which included cases of AFX and PDS grouped together, and for this reason, they were not included in this review. In that series, local recurrence was reported in 45 patients (14.1%) and distant metastasis in 33 patients (10.3%). After a complete analysis, the authors concluded that the aggressive behavior in these tumors grouped together depends on invasion beyond subcutaneous fat, tumor size greater than 2cm, immunosuppression, and presence of lymphovascular invasion. Another similar approach was taken by Cesinaro et al.28 in a series of 71 tumors with features consistent with AFX or PDS grouped together and with a follow-up time of between 17 and 125 months. Like the previous study, this one was not included in our review. Only 4 local recurrences were reported and there were no cases of metastasis. The spindle-cell morphology was associated with subcutaneous invasion and recurrence. Some authors consider AFX and PDS to be on the same spectrum of tumors, although they continue to use the individual terms to differentiate between them.33
With regard to subcutaneous invasion, we have shown in this review that the criteria for determining when it is present are by no means homogeneous. These variations of criteria when classifying the tumors have probably led to overdiagnosis of either AFX or PDS, depending on the series, with the subsequent impact on the rates of recurrence and/or metastasis. In the literature, AFX has been defined as a dermal neoplasm limited to the dermis or with minimal invasion of subcutaneous cellular tissue. The exact limit of the extent of invasion required to safely denote the tumor as AFX has never been uniformly established.34,35 According to the classification of the World Health Organization, initially it is specified that AFX is limited to the dermis, without invasion of the subcutaneous cellular tissue to then affirm in the differential diagnosis that lesions that resemble AFX but are larger or show substantial invasion of subcutaneous cellular tissue or beyond that, perineural invasion, lymphovascular invasion, or necrosis, should be classified as dermal pleomorphic sarcoma.36 On the other hand, and although this is an infrequent situation, cases have been described of AFX without subcutaneous invasion that lead to recurrence with invasion of deeper structures3 or even metastasis8; so though uncommon, it is not impossible.
Finally, diagnosis of AFX is currently considered to be performed based on an entire resection piece and not with partial biopsies, given that it is very important to assess the pattern of invasion, invasion of structures, and the other criteria for PDS.1 Although it was attempted to gather information systematically, the only study that specifies the number of cases diagnosed with involvement of the deep margin is that of Iglesias-Pena et al.,3 who also described 2 cases of spontaneous regression after an incisional biopsy. This is relevant because diagnosis of AFX and/or PDS, when the deep margin is positive, always generates a certain degree of uncertainty. It is necessary to establish some clear diagnostic criteria to classify tumors suggestive of AFX that regress after biopsy or incorrectly resected tumors, with involved margins that do not allow distinction between AFX and PDS, particularly when later margin widening gives a negative result.
ConclusionsThe series of AFX and PDS published in recent years classify these tumors mainly according to histopathologic features of subcutaneous invasion, tumor necrosis, perineural invasion, and lymphovascular invasion. The series of AFX that specify strict histopathologic criteria show that this is a benign neoplasm, but we lack larger series of patients with PDS to allow us to clearly establish what characteristics are associated with local recurrence or metastasis. Data published to date do not allow clear differentiation between these 2 tumors at other levels, and so some authors consider them the same entity that can follow a more aggressive course according to certain features of poor prognosis. Further studies are needed with large series of patients and careful description of histopathologic features to allow us to establish more rigorously factors of poor prognosis and thus help us choose the most appropriate treatment for our patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Iglesias-Pena N, Martínez-Campayo N, López-Solache L. Relación entre fibroxantoma atípico y sarcoma pleomórfico dérmico: histopatología de ambos y revisión de la literatura. Actas Dermosifiliogr. 2021;112:392–405.