Subacute cutaneous lupus erythematosus (SCLE) is a variant of photosensitive cutaneous lupus erythematosus that is often associated with anti-Ro antibodies.1
We present 3 cases of treatment-refractory SCLE that resolved satisfactorily with rituximab.
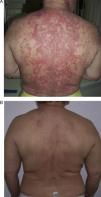
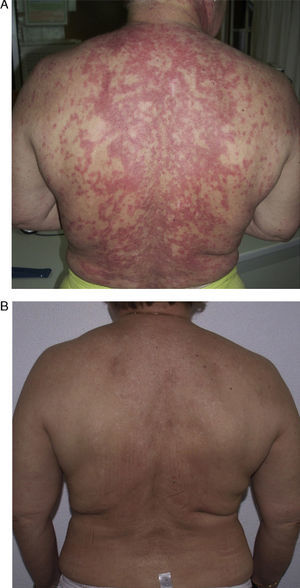
Patient 1 was a 54-year-old woman (smoker) diagnosed with SCLE. She had annular lesions on her back (Fig. 1A), upper chest, and arms. Testing for antinuclear antibody (ANA) was positive (titer, 1/640), with a homogeneous pattern; the results for other antibodies were negative.
The patient received high-dose prednisone (50mg/d), antimalarial agents (chloroquine 250mg/d and hydroxychloroquine 400mg/d) for 3 years, thalidomide 50-150mg/d for 16 months, cyclophosphamide 100mg/d, mycophenolate 500mg/d, etanercept (50mg, 2 doses per week), and intravenous immunoglobulins (2g/kg). Improvement was partial, and the patient experienced the following side effects: blurred vision (antimalarial agents), sensory axonal polyneuropathy (thalidomide), and leukopenia (cyclophosphamide).
In view of the lack of response, rituximab was started at a weekly dose of 375mg/m2 (4 doses) combined with prednisone 5-10mg/d. Remission was complete after 2 months. The same regimen was repeated annually (1g/15 d, 2 doses) for 4 years because of the appearance of new outbreaks, and control of the cutaneous lesions was excellent (Fig. 1B).
Patient 2 was a 37-year-old woman (smoker) with epilepsy, for which she was receiving carbamazepine but later switched to valproic acid. She had been diagnosed with SCLE at age 34 years and had annular lesions on her upper chest, back, and arms. Her ANA pattern was homogeneous (titer, 1/640), anti-La was positive, and testing for the remaining antibodies, including antihistone antibodies, was negative.
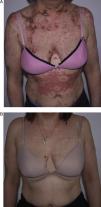
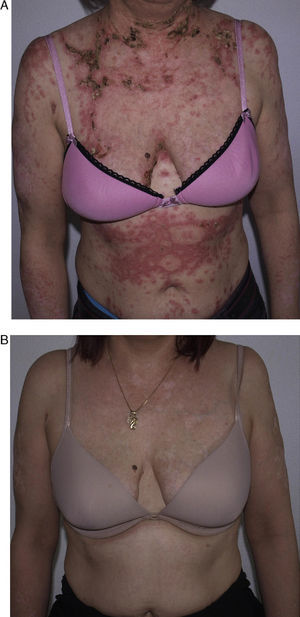
She had responded well to prednisone 50mg/d, hydroxychloroquine 400mg/d (18 months), and azathioprine 100mg/d, which was discontinued because of drug-induced leukopenia. Her skin lesions worsened considerably when azathioprine was stopped (Fig. 2A); therefore, rituximab was started at a weekly dose of 375mg/m2 (4 doses) combined with low-dose prednisone (10mg/d) and antimalarial agents. The patient progressed favorably, with no active lesions after 3 months. The regimen was repeated 1 year later (1g/15 d, 2 doses), and the patient remained free of active lesions during the 8 months following the last infusion of rituximab (Fig. 2B). Control of the disease was maintained with hydroxychloroquine 200-400mg/d.
Patient 3 was a 28-year-old woman who consulted with malar rash, asthenia, arthritis, low-grade fever, and annular lesions on the upper chest, back, and arms. Skin biopsy confirmed a clinical diagnosis of SCLE. A blood workup revealed reduced C3 and C4 levels, ANA (titer, 1/320) with a homogeneous pattern, and positive results for double-strand anti-DNA, anti-Ro, antiribonucleoprotein, and anti-Sm antibodies. The patient was diagnosed with systemic lupus erythematosus (SLE) and started treatment with prednisone 40mg/d combined with hydroxychloroquine 400mg/d for 6 months. The response was good, although her condition deteriorated when the dose of corticosteroid was reduced. Given our previous experience, we started treatment with rituximab at a weekly dose of 375mg/m2 (4 doses). Two months later, the patient's skin and clinical symptoms had improved considerably, although the malar rash persisted on the center of her face. Positive results were found for ANA, anti-DNA, and anti-Ro antibodies; the results for the remaining antibodies were negative. She is currently receiving prednisone at 10-20mg/d in combination with oral antimalarial agents.
Treatment of SCLE is based on strict photoprotection and administration of antimalarial agents as the first-line systemic approach. These drugs enable the disease to be controlled in up to 75% of cases.1,2 However, the remaining 25% require other types of systemic immunomodulatory or immunosuppressive therapy, which have variable efficacy and are not free of side effects.1,2
Rituximab is a chimeric monoclonal antibody that targets the CD20 antigen, which is expressed in pre-B cells, mature B cells, and memory cells, and leads to depletion of B lymphocytes.3 It is indicated mainly for B-cell lymphoproliferative disorders involving expression of CD20 (chronic lymphocytic leukemia and non-Hodgkin lymphoma),4 as well as in rheumatoid arthritis.5 Case series show its efficacy in pemphigus vulgaris, paraneoplastic pemphigus, cutaneous B-cell lymphoma, chronic graft-versus-host disease, and dermatomyositis.6
In SLE, depletion of B cells could lead to diminished production and expansion of autoreactive antibody-forming cells and to loss of the costimulatory signals delivered by B cells to T cells (CD40-CD40L interaction), thus blocking the antibody-dependent and antibody-independent tissue damage that occurs in lupus.3 Furthermore, the clinical efficacy and safety of rituximab has been reported in case series and open-label trials in patients with SLE. The duration of the drug's effectiveness varies, and it usually lasts from at least 5 months to around 14 months.3,7 However, few case reports show successful management of refractory cutaneous lupus with rituximab.
Risselada and Kallenberg8 reported the first 2 cases of refractory cutaneous lupus treated with rituximab in patients with SLE (2×1000mg/m2). The improvement in skin lesions was both marked and persistent. Uthman et al9 reported the case of a patient with SLE and extensive recalcitrant SCLE lesions that had been treated with rituximab (1g/wk, 2 doses) and improved considerably.9 Kieu et al10 presented the first case of SCLE that did not fulfill the criteria for SLE and was refractory to antimalarial agents and immunosuppressants. Treatment was with rituximab at a weekly dose of 375mg/m2 (4 doses). The same dose was administered a year later because the lesions recurred. The patient remained lesion-free for 2 years with maintenance therapy (375mg/m2 every 8 weeks).
We present 3 cases of refractory SCLE treated successfully with rituximab, which was well tolerated. Limited published experience and our findings in the present manuscript allow us to propose rituximab as an alternative for treatment of SCLE refractory to the usual approaches. However, larger-scale studies will enable us to set limits for this indication and establish the safety profile in this group of patients.
Please cite this article as: D.E. Cieza-Díaz, J.A. Avilés-Izquierdo, C. Ceballos-Rodríguez, R. Suárez-Fernández. Lupues eritematoso cutáneo subagudo refractario tratado con rituximab. Actas Dermosifiliogr.2012;103:555-557.