Cutaneous lupus erythematosus (CLE) is a chronic autoimmune skin disease that manifests in various morphological subtypes, all sharing a cytokine profile where type I interferon (IFN-I) plays a key role.1 These subtypes include acute CLE (ACLE), subacute CLE (SCLE), intermittent CLE (ICLE), and chronic CLE (CCLE), which itself includes discoid (DLE), chilblain lupus (CHLE), and lupus erythematosus panniculitis (LEP).1,2 Around 20% of CLE cases are associated with systemic involvement (systemic lupus erythematosus, SLE), and 70% of SLE patients eventually develop CLE.1,2 The risk of scarring lesions in CCLE makes early management essential. To date, no drugs are specifically approved for CLE, and treatments authorized for SLE are used off-label.1
Anifrolumab, a monoclonal antibody against the interferon alpha receptor subunit 1 (IFNAR1), was approved by the European Medicines Agency in February 2022 for SLE. In the secondary endpoints of the TULIP-2 clinical trial, a significant benefit for CLE was observed, achieving a 50% reduction in the Cutaneous Lupus Erythematosus Disease Area and Severity Index Activity (CLASI-A) score in 49% of patients.3 Since then, its safety and efficacy profile in the management of CLE in real-world clinical practice has been supported by the literature.4 In June 2023, the Spanish Ministry of Health approved funding for anifrolumab for moderate-to-severe active SLE with positive antinuclear antibodies (ANA). We present our experience with anifrolumab treatment in a case series of SLE patients with severe and refractory CLE involvement.
We conducted a single-center retrospective descriptive study on all cases of SLE with refractory cutaneous involvement treated with anifrolumab 300mg monthly from July 2023 through April 2024 in the Dermatology Department of a tertiary referral center in Spain. Study variables included sex, age, CLE subtype, systemic SLE involvement, autoantibodies, previous treatments, pre- and post-treatment CLASI-A, response time and follow-up, treatment optimization, and adverse events.
A total of 6 patients were included (Table 1), all of them women, with a median age of 50 years (range, 19–65). The median duration of SLE was 15 years (range, 2–42). The most prevalent systemic signs were hematologic (lymphopenia), polyarthritis, serositis, and lupus nephritis. All patients (100%) tested positive for ANA, with anti-Ro and anti-dsDNA being the most predominant specificities. The most widely observed CLE subtypes were DLE and ACLE, followed by SCLE and CHLE. The median number of previous treatments used was 4 (range, 4–12), with hydroxychloroquine, methotrexate, cyclosporine, azathioprine, and lenalidomide being the most frequent. Before starting treatment, patients completed the varicella-zoster virus vaccination regimen. All patients showed a rapid and remarkable remission of CLE lesions (Figs. 1 and 2) after a median of 2 months of treatment, that is, after 2 infusions of anifrolumab (range, 1–3). The median CLASI-A score dropped from 35 (range, 17–47) down to 2 (range, 0–5), and remission was maintained throughout the observation period. In 3 patients, treatment was optimized by spacing doses to every 2 months without lesion recurrence. The criterion for optimization was maintaining a CLASI score <3 points after the first 3 doses. No changes were observed in the other active SLE signs (hematologic and articular). One patient reported early recurrence of oral ulcers; 4, uncomplicated upper respiratory tract infections (URTIs); and 1 a reactivation of herpes simplex virus type 1 (HSV-1). No serious adverse events were observed or treatments discontinued.
Data from the 6 cases of systemic lupus erythematosus with severe cutaneous involvement in the study.
| Case report #1 | Case report #2 | Case report #3 | Case report #4 | Case report #5 | Case report #6 | Summary | |
|---|---|---|---|---|---|---|---|
| Age/gender | 19/F | 47/F | 53/F | 65/F | 44/F | 56/F | Median 50 (range, 19–65)100% F |
| CLE subtype | CHLE | SCLE, Rowell syndromeACLEDLELEP/LM | ACLEDLA | ACLEPeriorbital mucinosisDLA | DLESLA | DLEICLE | 50% ACLE50% DLA and SLA50% DLE16% SCLE16% CHLE16% ICLE16% LEP16% mucinosis |
| Systemic involvement (A: active/NA: inactive) | Lymphopenia (A)Oral aphthosis (A) | Polyarthritis (A) | Antiphospholipid syndromePleuropericarditis (NA)Lupus nephritis (NA) | Lymphopenia (A)Leukopenia (A)Pleuropericarditis (NA) | Polyarthritis (A) | Lupus nephritis (NA)ANCA vasculitis (NA)Secondary Sjögren's syndrome (A)Polyarthritis (A) | 50% Polyarthritis33% Lupus nephritis33% Lymphopenia33% Serositis16% ANCA vasculitis16% Antiphospholipid syndrome16% Sjögren's |
| Autoantibodies | ANAAnti-U1-RNP | ANAAnti-Ro | ANAAnti-dsDNA | ANAAnti-RoAnti-LaAnti-dsDNAAnti-U1-RNP | ANA | ANAAnti-SMAnti-Ro | 100% ANA50% Anti-Ro33% Anti-dsDNA33% Anti-U1-RNP16% Anti-SM16% Anti-La |
| Disease duration since SLE diagnosis (years) | 10 | 2 | 20 | 10 | 42 | 20 | Median 15 (range, 2–42) |
| Previous treatments | MTX, AZA, CP, HQ, Doxycycline, Apremilast, Colchicine, Dapsone, Nifedipine, Mepacrine, Sulfasalazine, Tofacitinib | MTX, CP, HQ, Lenalidomide | MMF, MTX, AZA, HQ | MTX, AZA, HQ, Leflunomide | CP, MTX, HQ, Ustekinumab, Apremilast, Baricitinib, Lenalidomide | HQ, MTX, AZA, CCF | 100% MTX, HQ 66%AZA50% CP33% Lenalidomide33% Apremilast33% JAK inhibitors16% Sulfasalazine16% Leflunomide16% Cyclophosphamide |
| CLASI-A before anifrolumab/CLASI-A at last visit | 33/0 | 47/2 | 17/2 | 49/1 | 23/5 | 20/0 | Median 28 (range, 17–47)/median 2 (range, 0–5) |
| Response time (months) (number of infusions) | 2 (2) | 2 (2) | 3 (3) | 1 (1) | 2 (2) | 1 (1) | Median 2 (range, 1–3) |
| Follow-up time (months) (number of infusions) | 10 (8) | 5 (3) | 3 (3) | 4 (3) | 2 (2) | 2 (2) | Median 3.5 (range, 2–10) |
| Treatment optimization (spacing every 2 months) | Yes (6th) | Yes (3rd) | No | Yes (3rd) | No | No | 50% optimized every 2 months (median of 3rd infusion) |
| Adverse events (treatment discontinuation) | None (No) | Rhinosinusitis Labial HSV-1 (No) | URTI (No) | URTI (No) | None (No) | URTI (No) | 66% URTI16% Labial HSV-1 33%None |
A: active extracutaneous manifestation; SLA: scarring lupus alopecia; DLA: diffuse lupus alopecia; ANA: antinuclear antibodies; Anti-dsDNA: anti-double-stranded DNA antibodies; Anti-SM: anti-Smith antibody; Anti-U1 RNP: anti-U1 ribonucleoprotein antibody; AZA: azathioprine; CCF: cyclophosphamide; CHLE: chilblain lupus erythematosus; CP: cyclosporine; HQ: hydroxychloroquine; iJAK: JAK kinase inhibitor; IVRA: upper respiratory tract infection; CLE: cutaneous lupus erythematosus; ACLE: acute cutaneous lupus erythematosus; DLE: discoid lupus erythematosus; ICLE: intermittent cutaneous lupus erythematosus; LEP: lupus erythematosus panniculitis; SCLE: subacute cutaneous lupus erythematosus; F: female; LM: lupus mastitis; MMF: mycophenolate mofetil; MTX: methotrexate; NA: non-active extracutaneous manifestation; NL: lupus nephritis; HSV-1: herpes simplex virus type 1.
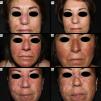
Images of cases with CLE of the DLE subtype prior to anifrolumab therapy (a) and after 2 infusions of anifrolumab 300mg/month (b). In all 3 cases, facial involvement due to DLE is observed, presenting as erythematous, edematous, and scaly infiltrated plaques. After treatment with anifrolumab, the plaques appear without erythema or infiltration, showing hypopigmentation and scarring. (Image set 1 corresponds to case report #6 in the table, image set 2 to case report #2, and image set 3 to case report #5.)
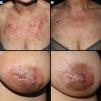
Images of cases with CLE of the ACLE and LEP subtypes prior to anifrolumab therapy (a) and after 2 infusions of anifrolumab 300mg/month (b). Image set 1 corresponds to case report #4 in the table and shows ACLE lesions presenting as erythematous and edematous plaques in a sun-exposed area (décolletage), with acute onset, that heal without scarring after treatment. Image set 2 corresponds to case report #2 and shows lupus mastitis (LEP) lesions characterized by indurated, infiltrated, scaly plaques with deep, fissured involvement that resolve leaving a scar after treatment.
The IFN-I family includes 5 classes that bind and signal through IFNAR: IFN-α, IFN-β, IFN-ω, IFN-κ, and IFN-ɛ.5 Anifrolumab competitively inhibits the binding of IFN-I to IFNAR1. IFN-α, secreted mainly by CD123+ dendritic cells, acts as a hinge or linking point between innate and adaptive immunity in CLE pathogenesis.6 Until now, belimumab and rituximab were the only biological drugs approved for SLE, both targeting B lymphocytes. While they have demonstrated efficacy in musculoskeletal and visceral signs of SLE, their cutaneous response was limited. Anifrolumab, on the other hand, seems to have a better efficacy profile for cutaneous involvement vs other SLE signs.3,7 Blocking IFN-I has become a promising avenue in CLE treatment.5
The safety and efficacy profile and data from our study are consistent with those observed in clinical trials and real-world studies.4,8–10 Anifrolumab was effective for treating all variants of refractory CLE, notably for its rapid onset of action.4,6 As noted in the literature, adverse events were mild and self-limiting, with URTIs and HSV-1 reactivation being the most common finding.9 We also highlight the possibility of optimizing treatment by spacing doses while maintaining complete remissions over time.
Conflict of interestThe authors declare that they have no conflict of interest.