Evidence for secukinumab and bimekizumab in hidradenitis suppurativa (HS) primarily comes from randomized clinical trials with selected patient populations. Conversely, real-world evidence (RWE) studies reflect broader patient demographics and clinical practice. This study aims to summarize RWE on the safety and efficacy profile of secukinumab and bimekizumab for HS through a systematic review and meta-analysis.
MethodsWe conducted a comprehensive search using the Medline and Scopus databases. Eligible studies reported RWE on secukinumab or bimekizumab in HS. Furthermore, we conducted a meta-analysis to estimate the proportion of patients achieving HiSCR with secukinumab.
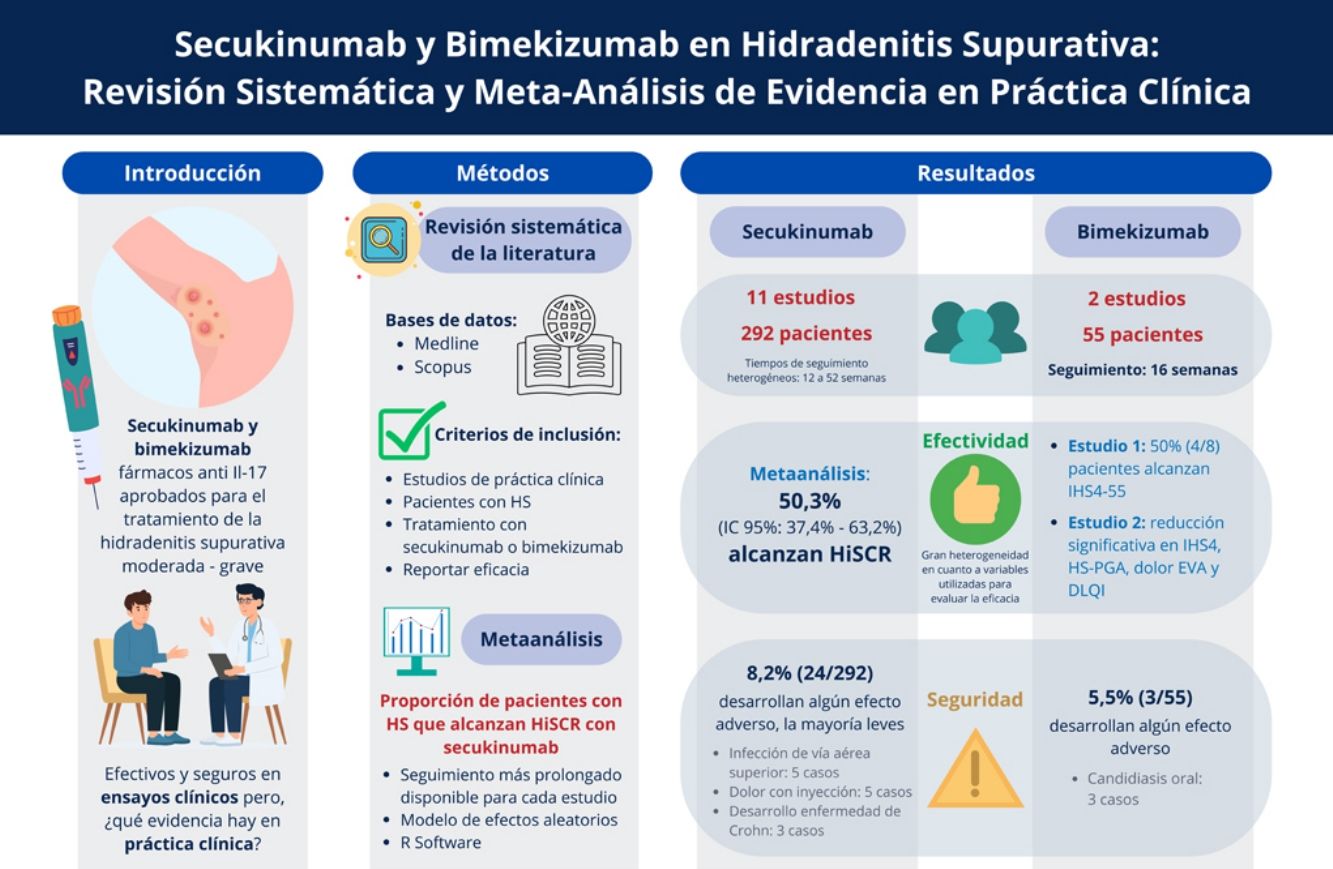
ResultsA total of 13 studies were included, with 347 HS patients. The meta-analysis showed that 50.31% (95%CI, 37.41–63.18%) of patients on secukinumab achieved HiSCR at the longest follow-up available. While HiSCR was not reported for bimekizumab studies, significant improvements in IHS4 and pain-NRS were observed. Both drugs were well-tolerated, with adverse event rates of 8.22% for secukinumab and 5.45% for bimekizumab, most being mild and manageable.
ConclusionsIL-17 inhibitors provide moderate response rates and are valuable options for patients refractory to other therapies, with low incidences of mild adverse events. More RWE studies are essential to better understand their safety and efficacy profile.
Hidradenitis suppurativa (HS) presents a considerable treatment challenge, and many patients remain with inadequate disease control despite the currently available therapeutic options. Recent advances in the therapeutic approach to HS have emerged with the development of cytokine inhibitors and small molecule drugs. These advancements are largely due to a deeper understanding of the disease's pathophysiology, especially the role of the Th17 inflammatory pathway.1,2 Currently, 2 IL-17 inhibitors, secukinumab and bimekizumab, have been approved for the treatment of HS, following positive outcomes in phase III randomized clinical trials.3,4 Additionally, other IL-17 inhibitors, including sonelokimab, izokibep, and CJM112, are currently being investigated in both phase II and phase III clinical trials.5–8
While clinical trials are essential for evaluating the safety and efficacy profile of new treatments, they are conducted under controlled conditions with carefully selected patient populations. This controlled environment often does not fully represent the broader and more diverse patient populations encountered in everyday clinical practice. As a result, the findings from these trials may not always be generalizable to real-world settings. Real-world studies, on the other hand, include a broader range of patients and reflect the complexities and variability of actual clinical practice, potentially offering more relevant and applicable information for routine care. This evidence is crucial for bridging the gap between clinical trial results and the actual effectiveness and safety of treatments in diverse patient populations.9,10
Even before the official approval of secukinumab and bimekizumab, these drugs were used in real-world clinical practice as compassionate options for patients resistant to adalimumab, due to the limited therapeutic alternatives available.11–13 Following their approval, ongoing studies continue to evaluate their effectiveness and safety in real-world settings.
The aim of this study is to systematically review and analyze the available real-world evidence (RWE) on the safety and efficacy profile of secukinumab and bimekizumab in patients with HS through a comprehensive systematic review and meta-analysis of the current literature.
Material and methodsProtocolThis systematic review was conducted in full compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.14 It was officially registered in the International Prospective Register of Systematic Reviews (PROSPERO) database with identification No. CRD42024576817.
Search strategyWe conducted a comprehensive literature search across Medline and Scopus databases, reviewing all titles available up to July 2024. The search algorithm was “hidradenitis suppurativa” AND (“secukinumab” OR “bimekizumab”). Bibliographic references from eligible articles were reviewed individually to identify additional relevant studies.
Inclusion and exclusion criteriaStudies were considered eligible for inclusion if they met the following criteria:
- A)
Written in English language.
- B)
Epidemiological studies conducted in a real-world setting, including single-arm studies, retrospective studies, cross-sectional studies, observational studies, and case series of at least 5 participants.
- C)
Documented the safety and/or efficacy profile of secukinumab or bimekizumab for the treatment of HS.
- D)
Reported at least one of the following outcomes: hidradenitis suppurativa clinical response (HiSCR), a 55% reduction in the International Hidradenitis Suppurativa 4 score (IHS4-55), Physician Global Assessment (PGA), Hidradenitis Suppurativa Physician Global Assessment (HS-PGA), Dermatology Life Quality Index (DLQI), or adverse events.
Randomized controlled trials were excluded, as the objective of the review is to gather RWE. Case series with fewer than 5 participants, case reports, and reviews were excluded as they may lack sufficient data to meaningfully contribute to the review's objectives.
Study selectionThree authors (SH, DMB, ASM) independently screened all titles and abstracts to identify relevant studies. Relevant studies underwent full-text reading to determine their final inclusion or exclusion. Any disagreement was resolved by discussion with a fourth author (AML).
Risk of bias and quality assessmentThe level of evidence for the included articles was determined using the 2011 Levels of Evidence guidelines from the Oxford Centre for Evidence-Based Medicine.15
Three reviewers (SH, DMB, ASM) independently assessed the risk of bias and quality of studies using the National Institutes of Health quality assessment tool for before-after (pre-post) studies with no control group and for case series studies.16 Discordances were resolved by discussion with a fourth author (AML).
Data collectionThree authors (SH, DMB, ASM) independently collected the following data from included studies: authors’ names, publication year, country of origin, study design, sample size, demographic characteristics of participants, inclusion criteria, prior anti-TNFa exposure, treatment protocols, duration of follow-up, outcome measures, efficacy data, and adverse events.
Data synthesis and statistical analysisBasic descriptive statistics were extracted and summarized. Continuous variables are expressed as mean and standard deviation (SD), and qualitative variables as relative and absolute frequency distributions. A weighted mean was calculated for continuous variables, with each study mean weighted by its sample size. A pooled standard deviation was computed using variance data from studies with reported SDs.
A meta-analysis of proportions was performed to determine the overall rate of HS patients achieving HiSCR with secukinumab. For studies with multiple time points, data from the longest follow-up were used. HiSCR was calculated using the initial sample size of each study, treating patients who did not complete follow-up as non-responders. A random effects model was applied to combine response proportions, adjusting for study variability and precision using inverse variance weighting. The overall proportion, standard error, and 95% confidence interval were computed to estimate the percentage of patients achieving HiSCR. All analyses were conducted using R software, version 4.4.1.17
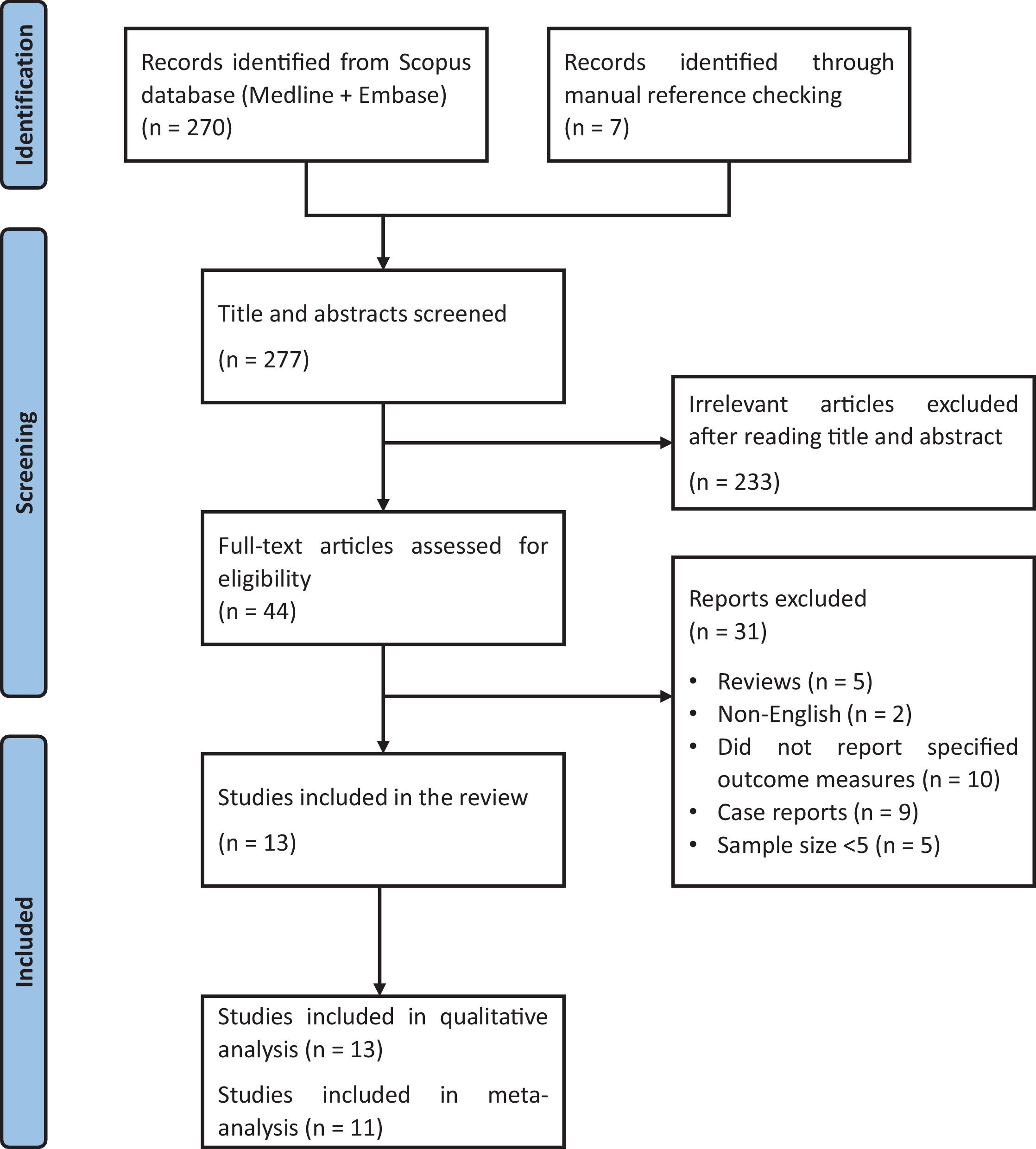
ResultsStudy characteristicsWe identified a total of 277 references, 269 through database searches and 7 via manual reference review. After title and abstract screening, a total of 44 articles were fully read, and 13 studies were considered eligible for systematic review18–30 (Fig. 1).
Among these 13 studies, 1118–28 focused on the effectiveness of secukinumab in HS patients, while 229,30 studied the efficacy of bimekizumab (Tables 1 and 2 and Supplementary Tables 3 and 4). Studies included 2 open-label uncontrolled trials,18,19 9 retrospective observational studies,20–28 ambispective observational study,30 and 1 case series.29 The studies were conducted between 2019 and 2024 and involved a total of 347 HS patients on an IL-17 inhibitor: 292 received secukinumab and 55, bimekizumab. All studies originated from high-income countries, including 6 from Spain,22–24,27,29,30 4 from Italy,21,25,26,28 2 from the United States,18,19 and 1 from France.20
Summarized data on secukinumab studies.
| Author, year and country of publication | Study type | Drug and dosage | Sample size | Time of response assessment | Outcome measures reported | Effectiveness (primary outcomes: HiSCR, IHS4-55) | Adverse events |
|---|---|---|---|---|---|---|---|
| Prussick et al.18USA, 2019 | Open label trial with no control group | Secukinumab300mg weekly for 5 weeks, then e4w | 9 | Week 24 | HiSCR, Sartorius score, DLQI, inflammatory lesion count | HiSCR- Week 24: 78% (7/9) | No SAEs reported.5 cases of self-resolving upper respiratory tract infections |
| Casseres et al.19USA, 2020 | Open label trial with no control group | Secukinumab300mg weekly for 5 weeks, then e4w (n=9) or e2w (n=11) | 20 | Weeks 12 and 24 | HiSCR, Modified Sartorius score, DLQI, AN50, AN75, AN100 | HiSCR- Week 12: 65% (13/20)- Week 24: 70% (14/20) | No SAEs reported. |
| Reguiai et al.20France, 2020 | Retrospective observational study | Secukinumab300mg weekly for 5 weeks, then e4w | 20 | Week 16 | HiSCR, HS-PGA | HiSCR- Week 16: 75%, (15/20) | Two patients developed Crohn disease after 3 and 5 months, with no personal or family history of inflammatory bowel disease; secukinumab was discontinued in these patients |
| Ribero et al.21Italy, 2021 | Multicenter retrospective observational study | Secukinumab300mg weekly for 5 weeks, then e4w | 31 | Week 28 | HiSCR, AN count, IHS4, DLQI | HiSCR:- Week 5: 10% (3/31)- Week 16: 26% (8/24)- Week 28: 41% (7/17) | One case of acne-like eruption of the face |
| Melgosa et al.22Spain, 2022(JEADV) | Retrospective observational study | Secukinumab300mg weekly for 5 weeks, then e4w until week 24, then e2w (10/23), e3w or e4w (13/23) | 23 | Weeks 16, 24, 36 and 52 | HiSCR, IHS4 | HiSCR- Week 16: 73.9% (17/21)- Week 24: 71.4% (15/21)- Week 36: 71.4% (10/14)- Week 52: 83.3% (10/12) | No SAEs.Four cases of mild local pain during secukinumab administration, without requiring drug withdrawal |
| Melgosa et al.23Spain, 2022(Actas) | Retrospective observational study | Secukinumab300mg weekly for 5 weeks, then e4w. | 14 | Week 12 | HiSCR, PGA | HiSCR:- Week 12: 85% (12/14) | No SAEs reported. |
| Fernández-Crehuet et al.24Spain, 2023 | Multicenter retrospective observational study | Secukinumab300mg weekly for 5 weeks, then e4w (35/47) or e2w (12/47) | 47 | Week 16 | HiSCR, IHS4, IHS455, Pain NRS, inflammatory nodules count, abscesses count, inflamed or draining tunnels count | HiSCR- Week 16: 48.9% (23/47)IHS4-55- Week 16: 40.4% (19/47) | One case of oral candidiasis, one case of previous psoriasis worsening, one case of articular pain and inflammation.The last two led to treatment discontinuation. |
| Repetto et al.25Italy, 2023 | Retrospective observational study | Secukinumab300mg weekly for 5 weeks, then e4w | 16 | Weeks 12, 24, 36 and 48 | HiSCR, IHS4, DLQI | HiSCR- Week 12: 6.3% (1/16)- Week 24: 37.5% (6/16)- Week 36: 37.5% (6/16)- Week 48: 31.3% (5/16) | No SAEs reported. |
| Martora et al.26Italy, 2024 | Retrospective observational study | Secukinumab300mg weekly for 5 weeks, then e4w | 21 | Week 52 | HiSCR, IHS4, DLQI, Pain VAS | HiSCR- Week 16: 57.1% (8/14)- Week 52: 71.4% (10/14) | One case of pneumonia at week 12 |
| Haselgruber et al.27Spain, 2024 | Multicenter retrospective observational study | Secukinumab300mg weekly for 5 weeks, then e2w (14/67) or e4w (53/67) | 67 | Weeks 16 and 24 | HiSCR, IHS4, IHS4-55, Pain VAS, inflammatory nodules count, abscesses count, inflamed or draining tunnels count | HiSCR- Week 16: 43.28% (29/67)- Week 24: 41.79% (28/67)IHS4-55- Week 16: 35.82% (24/67)- Week 24: 44.78% (30/67) | 6 adverse events at week 16: Oral candidiasis, Psoriasis worsening, two cases of articular pain and inflammation, Crohn's disease onset, Headaches1 adverse event at week 24: pain at the injection site |
| Rocuzzo et al.28Italy, 2024 | Retrospective observational study | Secukinumab300mg weekly for 5 weeks, then e4w | 24 | Weeks 12, 24, 36 and 48 | HiSCR, IHS-4, IHS4-55, DLQI | HiSCR:- Week 12: 25% (6/24)- Week 24: 56.5% (13/23)- Week 36: 40.9% (9/22)- Week 48: 28.5% (6/21)IHS4-55:- Week 12: 8.3% (2/24)- Week 24: 54.2% (13/23)- Week 36: 31.8% (7/22)- Week 48: 28.5% (6/21) | One case of headache that led to drug discontinuation |
HS: hidradenitis Suppurativa; IHS4: International Hidradenitis Suppurativa Severity Score System; HiSCR: Hidradenitis Suppurativa Clinical Response; IHS4-55: A reduction of at least 55% from the baseline IHS4 score; e4w: Every 4 Weeks; e2w: Every 2 Weeks; AN: abscesses and inflammatory nodules; DLQI: Dermatology Life Quality Index; NRS: Numerical Rating Scale; VAS: Visual Analog Scale; HS-PGA: Hidradenitis Suppurativa Physician Global Assessment; SAEs: Serious Adverse Events.
Summarized data on bimekizumab studies.
| Author, year and country of publication | Study type | Drug and dosage | Sample size | Time of response assessment | Outcome measures reported | Effectiveness (primary outcomes) | Adverse events |
|---|---|---|---|---|---|---|---|
| Ureña-Paniego et al.29Spain, 2024 | Case series | Bimekizumab. Dosing not reported. | 15 | Week 16 | AN count, Draining tunnel count, IHS4, IHS4-55, pain VAS, pruritus VAS, malodour VAS, discharge VAS, patient global impression | IHS4-55:- Week 16: 50% (4/8) | No SAEs reported. |
| Mansilla-Polo et al.30Spain, 2024 | Multicentric ambispective observational study | Bimekizumab 320mg, either e2w or e4w | 40 | Week 16 | IHS4, VAS Pain, HS-PGA, DLQI | – | 3 cases of oral candidiasis, mild and localized, successfully managed with antifungal therapy |
HS: hidradenitis suppurativa; IHS4: International Hidradenitis Suppurativa Severity Score System; IHS4-55: a reduction of at least 55% from the baseline IHS4 score; e4w: Every 4 Weeks; e2w: Every 2 Weeks; AN: abscesses and inflammatory nodules; DLQI: Dermatology Life Quality Index; NRS: Numerical Rating Scale; VAS: Visual Analog Scale; HS-PGA: Hidradenitis Suppurativa Physician Global Assessment; SAEs: Serious Adverse Events.
The risk of bias and methodological quality of the 2 open-label uncontrolled trials, 9 retrospective observational studies, and 1 ambispective observational study were assessed using the National Institutes of Health quality assessment tool for before-after (pre-post) studies with no control group.16 Two studies were rated as being of poor quality,18,22 5 as being of fair quality,19–21,23,26 and 5 as being of good quality.24,25,27,28,30 Common methodological issues included the following: (1) outcome assessors were not blinded to participants’ interventions (n=13); (2) it was unclear whether study participants were representative of the target population eligible for the intervention (n=8); (3) studies did not report whether all eligible participants meeting prespecified entry criteria were enrolled (n=9); and (4) loss to follow-up of 20% or greater after baseline (n=6) (Supplementary Table 1).
The risk of bias and methodological quality of the single case-series study29 was rated as good using the National Institutes of Health quality assessment tool for case-series studies16 (Supplementary Table 2).
Outcome measuresThe studies used 20 different outcome measures to evaluate treatment effectiveness. The most widely used tools included HiSCR (n=11), IHS-4 (n=9), DLQI (n=7) and Pain VAS or NRS (n=5) (Supplementary Table 5).
SecukinumabStudies and patientsWe identified a total of 11 RWE studies,18–28 involving 292 patients on secukinumab for HS. Most studies included patients with moderate-to-severe HS (defined as Hurley II or III) who had previously failed or had contraindications to an anti-TNFa drug (Supplementary Table 3).
The cohort included 137 men (46.92%) and 155 women (53.08%). Regarding HS severity at baseline, Hurley stage information was available for 246 patients: 2.03% (5/246) were Hurley I HS, 45.12% (111/246) Hurley II, and 52.85% (130/246) Hurley III. IHS4 data was available for 229 patients, showing a mean baseline IHS4 score of 17.57±9.73. Additionally, information on prior exposure to anti-TNF drugs was available for 229 patients, 89.96% (206/229) of whom had experienced primary or secondary failure with an anti-TNF before starting secukinumab.
The secukinumab maintenance regimen was 300mg every 4 weeks (e4w) for 87.33% (255/292) of patients and 300mg every 2 weeks (e2w) for the remaining 12.67% (37/292).
Most studies did not report on whether concomitant therapies, such as topical or systemic medications, minor procedures like incision and drainage or intralesional corticosteroid administration, or major surgery were allowed or prohibited, nor did they specify if patients received any of these therapies alongside secukinumab. In the study by Repetto et al.,25 75% (12/16) of patients used topical clindamycin. In the studies by Fernandez-Crehuet et al.24 and Haselgruber et al.,27 concomitant drugs and surgical procedures alongside secukinumab were allowed. In the first study,24 27.66% (13/47) of patients received concomitant antibiotics or oral corticosteroids, and 2 patients underwent major surgery for HS. In the second study,27 32.84% (22/67) of patients received concomitant medication (including systemic antibiotics, oral corticosteroids, spironolactone, antiandrogenic oral contraceptive pills, or acitretin) and 6 patients underwent surgery for HS. Information on other minor procedures was not detailed.
EffectivenessThe effectiveness of secukinumab was evaluated at various follow-up points, ranging from 12 to 52 weeks. Most studies (n=6)18–20,23,24,27 assessed effectiveness at 24 weeks (∼6 months) or earlier.
IHS4-55 was reported in only 3 studies,24,27,28 with data available for 135 patients: 40.74% (55/135) of patients achieved IHS4-55 16 and 48 weeks into therapy.
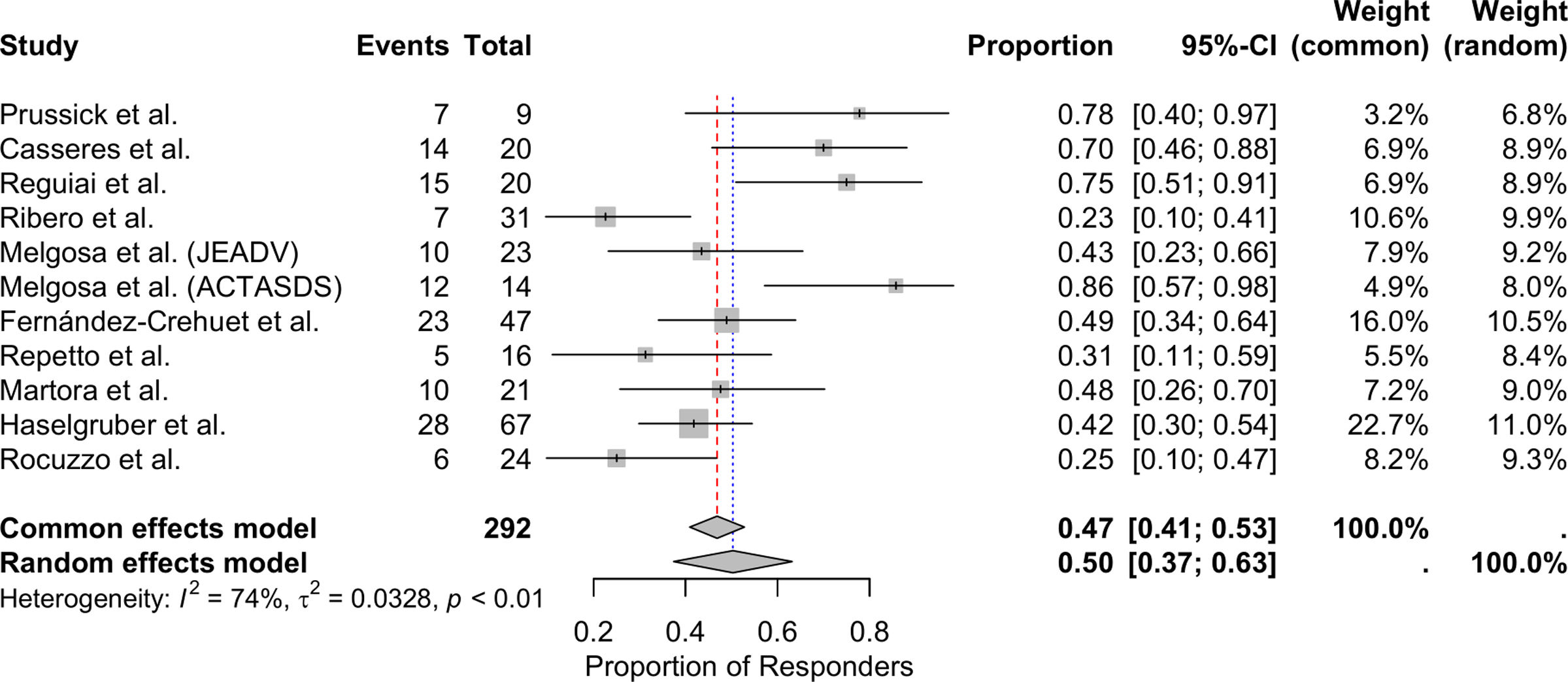
HiSCR was reported in all 11 studies.18–28 Considering only the time point with the longest follow-up in each study, data were available for 257 patients, 137 of whom (53.31%) achieved HiSCR 12 and 52 weeks into therapy. The study with the largest patient cohort,27 including 67 patients, reported a HiSCR of 41.79% (28/67) on week 24.
The 2 studies with the longest follow-up period,22,26 both of 52 weeks (∼one year), reported HiSCR rates of 83.3% (10/12)22 and 71.4% (10/14).26 Both studies reported data exclusively from patients who completed the 52-week follow-up period, not including in the final analysis patients who discontinued secukinumab for any reason before week 52.
In 5 studies,19,22,25,27,28 HiSCR was evaluated at multiple time points throughout the follow-up period. Melgosa et al.22 and Martora et al.26 found that the efficacy profile of secukinumab increased over time without declining during the follow-up. In contrast, Repetto et al.25 and Rocuzzo et al.28 observed that the efficacy profile increased during the initial months peaking at 6 months, but then experienced a subsequent decline.
Meta-analysis of patients achieving HiSCRThe meta-analysis, conducted using a random-effects model, showed that the overall proportion of patients with HS achieving HiSCR with secukinumab was 50.31% (95%CI, 37.41–63.18%). Significant heterogeneity was observed among the included studies, with an I2 statistic of 73.7%, indicating substantial variability in response rates across studies (Fig. 2).
Dosing regimens and effectivenessIn most studies,18,20,21,23,25,26,28 the standard secukinumab maintenance regimen, 300mg e4w, was used. However, in 4 studies,19,22,24,27 some patients received an intensified regimen of 300mg e2w.
Casseres et al.19 did not report on whether there were any differences in the efficacy profile between the 2 dosing regimens. Fernández-Crehuet et al.24 and Haselgruber et al.27 observed no significant difference in the efficacy profile between the standard and the intensified regimens. In the study by Melgosa et al.,22 all 23 patients initially took 300mg e4w. However, after 24 weeks, 13 patients had their regimen intensified to e3w or e2w due to an inadequate response. This adjustment led to improved efficacy in 10 of the 13 patients.
Predictors of responseIn 5 studies,20,21,24,27,28 researchers investigated whether any clinical variables influenced the response to secukinumab (Supplementary Table 6).
Two of these studies20,21 didn’t find any variable that could influence the response to the treatment. One study24 reported that being a woman and having a lower therapeutic burden (defined as the total number of previous cycles of systemic treatments and surgeries for HS) were associated with a higher likelihood of achieving HiSCR with secukinumab. Another study27 found that patients with a therapeutic burden of ≤5 were more likely to achieve both HiSCR and IHS4-55. In the last study,28 the primary factor influencing the response to secukinumab was the disease severity, as indicated by baseline IHS4 score and Hurley stage.
SafetyAdverse events were reported in 8.22% (24/292) patients on secukinumab. The most common adverse events observed were self-limiting upper respiratory tract infections (5 cases) and mild pain at the injection site (5 cases). Additionally, three cases of Crohn's disease onset were reported, which led to secukinumab discontinuation.
BimekizumabStudies and patientsWe identified 2 RWE studies29,30 with 55 HS patients on bimekizumab. The patient cohort included 33 men (60%) and 22 women (40%). Among these patients, 16 (29.1%) were Hurley II, and 39 (70.9%) were Hurley III. The mean baseline IHS4 score was 26.49±12.58. Additionally, 92.7% (51/55) of patients had prior exposure to biologic drugs, although the specific drugs were not specified (Supplementary Table 4).
In the first study29 the dosing regimen for bimekizumab was not specified, and it was not reported whether concomitant therapies were allowed or prohibited. In the second study,30 patients on bimekizumab 320mg every 2 weeks (Q2W) or every 4 weeks (Q4W); however, the number of patients in each dosing group was not specified. Additionally, 53% of patients received concomitant therapy, most commonly topical clindamycin, followed by oral clindamycin. No information was provided regarding concomitant surgeries or other minor procedures.
EfficacyBoth studies29,30 evaluated the efficacy profile of bimekizumab 16 weeks into therapy. Neither study reported HiSCR. Only 1 study29 documented IHS4-55, reporting a 50% (4/8) response rate by week 16. The other study30 documented significant reductions in mean IHS4, HS-PGA, pain VAS, and DLQI scores among the 34 patients who completed follow-up.
SafetyAdverse events were reported in 3/55 (5.45%) patients, all of whom experienced mild oral candidiasis, which was effectively managed with antifungal therapy.
DiscussionThis systematic review compiles the available evidence on the use of the 2 IL-17 inhibitors approved for the treatment of HS, secukinumab and bimekizumab, in real-world clinical practice. A total of 13 studies were included, involving 292 HS patients on secukinumab and 55 patients on bimekizumab. Of those on secukinumab, the meta-analysis found that 50.31% achieved HiSCR at the longest follow-up available. Patients on bimekizumab showed significant reductions in IHS4 and pain VAS scores, among other measures.
Although bimekizumab and secukinumab are both IL-17 inhibitors, they differ in their mechanisms of action: bimekizumab inhibits both IL-17A and IL-17F, while secukinumab targets only IL-17A. Although IL-17F is a weaker inducer of proinflammatory cytokines, it is upregulated in early and chronic HS lesions, contributing to ongoing inflammation. Bimekizumab dual inhibition is theorized to provide better control of inflammation in HS compared to secukinumab.31 However, a direct comparison of the efficacy between bimekizumab and secukinumab is not currently possible, as no head-to-head studies have been conducted.
This review suggests that while both drugs may offer similar benefits, the significant imbalance in the number of studies and patients treated with each drug limits meaningful comparisons. Of the 13 studies reviewed, 11 focused on secukinumab, while only 2 examined bimekizumab, which reduces the generalizability of conclusions about bimekizumab. Consequently, the current evidence is insufficient to determine their comparative effectiveness. Future research should address this gap by conducting direct comparisons to identify specific patient populations or clinical scenarios where one drug may provide superior outcomes.
Overall, real-world data on IL-17 inhibitors for treating HS remains limited, particularly due to the small sample sizes of the included studies. This limitation is understandable given the low prevalence of HS and the relatively recent adoption of these therapies. Despite their small scale, these studies provide valuable insights into how IL-17 inhibitors are used in everyday clinical practice, offering a complementary perspective to that of RCTs.
Another important point that should be taken into consideration is that RCTs and RWE studies have several key distinctions. SUNNY and BE HEARD trials established both minimum and maximum thresholds for the number of inflammatory lesions, whereas most RWE studies did not impose such criteria. Another major difference lies in the severity of HS among patients: most participants in the RCTs had moderate HS, classified as Hurley II (59.04% in SUNNY and 55.72% in BE HEARD trials). In contrast, RWE studies predominantly included patients with severe HS, classified as Hurley III (52.85% in secukinumab studies and 70.9% in bimekizumab studies). The most notable difference, however, is the previous exposure to biologic therapies. In the RCTs, most patients were biologic-naive, with only 24% in the SUNNY and 19% in the BE HEARD trials having prior exposure to a biologic drug. On the other hand, about 90% of patients from the RWE studies had previously received at least one biologic agent for HS, with most experiencing treatment failure or, less frequently, adverse events. Patients included in RWE studies generally had more severe disease and had previously failed at least one biologic therapy, which may suggest reduced treatment effectiveness compared with patients enrolled in RCTs However, the efficacy profile reported is quite similar. In the SUNNY trials, HiSCR rates at week 16 ranged from 42% to 46%, comparable to the rates reported in the RWE studies. Notably, RWE studies on bimekizumab did not use HiSCR to report the drug's efficacy, which limits direct comparison with the results from the BE HEARD trials, where HiSCR was 45–54% at week 16.3,4
Although HiSCR has become a widely used tool for assessing treatment efficacy in HS, it has some limitations.32,33 It defines a response as a 50% reduction in abscesses and nodules, dynamic lesions that may resolve spontaneously over time. As a result, HiSCR might categorize a patient as responder merely because their acute lesions have diminished, while ignoring persistent, static lesions such as draining tunnels. This may contribute to the observed high rates of HiSCR responses in patients receiving placebo in RCTs.3,4 Additionally, HiSCR only measures changes at specific timepoints and does not reflect the overall course of the disease, which alternates between flare-ups and periods of stability. A new tool, the IHS4-55, defines a response as a reduction of at least 55% in the baseline IHS4 and addresses the issue of draining tunnels by including them and emphasizing their impact in the formula.34 However, IHS4-55 still does not capture the fluctuant course of HS. A recently proposed tool, the cumulative-IHS4, aims to address this by considering the number of lesions over the entire evaluation period rather than at specific timepoints. Nevertheless, this tool requires further evidence and validation.35
Biologic drugs alone often do not provide sufficient control of the disease. Adjuvant therapy, such as systemic antibiotics for flare-ups, incision and drainage or intralesional corticosteroid injections for isolated symptomatic lesions, and surgery for tunnel removal, are often necessary.36,37 However, most of the studies reviewed did not specify whether patients received these additional therapies alongside the IL-17 inhibitor. In contrast, the SUNNY and BE HEARD trials allowed concomitant therapies, either chronic or as bailout treatments.3,4 The use of these additional therapies complicates the evaluation of the biologic drug efficacy profile, as capturing and analyzing the data becomes challenging and there is no standardized method for assessing effectiveness when adjunctive treatments are involved.
Regarding the safety profile, IL-17 inhibitors are generally well-tolerated. Common side effects observed in both RCTs and RWE studies included upper respiratory infections, headaches, and oral candidiasis.3,4 These side effects are usually mild and manageable with standard treatments, rarely requiring discontinuation of the medication. Inflammatory bowel disease (IBD) cases were reported in both RWE studies (3 cases) and RCTs (10 cases). Patients with HS are already at a higher risk for IBD, and IL-17A inhibition has been associated with this condition.38–41 Since IBD can be a serious issue that often requires stopping the IL-17 inhibitor, careful monitoring for this side effect is essential.
An important source of heterogeneity among the reviewed studies was the time of response assessment, which ranged from 12 to 52 weeks. This variability makes it challenging to compare studies and draw clear conclusions about the overall effectiveness of IL-17 inhibitors. Additionally, most studies evaluated efficacy 16–24 weeks into therapy, which may not be sufficient to fully assess the long-term response to IL-17 inhibitors.
Another important aspect is how studies handled missing data from patients who did not complete follow-up. When patients drop out or discontinue the drug, often due to lack of efficacy, there are 2 main approaches to account for these cases: include the entire study population by carrying forward the last recorded data, or only include patients who completed follow-up. Excluding patients who stopped treatment early, potentially due to inefficacy, could result in inflated response rates. In our meta-analysis, HiSCR was calculated using the initial sample size of each study, treating patients who did not complete follow-up as non-responders. This method provides a more conservative estimate of treatment efficacy by acknowledging that discontinuation may be due to inefficacy. However, it assumes that all patients who did not complete the study are non-responders, which may not fully reflect their actual response.
As discussed earlier, this review has several limitations, including the limited number of studies examining bimekizumab, small sample sizes, variability in outcome measures, different time of response assessments, varying methods for handling missing data, and insufficient information on adjuvant therapies used along the biological drug. These factors limit the ability to synthesize evidence effectively and affect the overall quality of the results.
In conclusion, the findings from RWE studies fairly align with those from RCTs. Although IL-17 inhibitors show moderate response rates, not achieving the success observed in other inflammatory diseases, they can be considered a valuable therapeutic option for patients refractory to other treatments. These drugs generally have a low rate of adverse events, which are usually mild. Some remaining issues to be addressed include identifying specific disease types or patient profiles most likely to benefit from these drugs and developing outcome measures that accurately capture all treatments used and reflect disease control over the entire treatment period.
Conflict of interestThe authors declare that they have no conflict of interest.
This systematic review is part of the doctoral thesis conducted by Sofía Haselgruber.
We would like to express our most sincere gratitude to all the researchers and authors whose work has contributed to this systematic review. Their valuable insights and findings have greatly enriched our understanding of the topic. We also appreciate the efforts of the research teams and institutions that conducted the studies included in this review. Your dedication to advancing knowledge in this field is commendable.