Pytiriasis lichenoides (PL) is a spectrum of uncommon inflammatory conditions with considerable clinical overlap. These disorders seem to affect equally both sexes and are more prevalent in children and young adults.1,2 Besides pytiriasis lichenoide chronica (PLC), the most common presentation,3 PL also encompasses pityriasis lichenoides et varioliformis acuta (PLEVA) and febrile ulceronecrotic Mucha-Habermann disease (FUMHD). PLEVA has a more acute onset than PLC, can be accompanied by systemic symptoms and presents with erythematous scaly papules that can develop central vesicopustules and hemorrhagic crusts, with subsequent varioliform scars4; FUMHD has exuberant systemic symptoms and progresses rapidly to necrotic lesions, ulcers, and blisters, with a higher mortality rate.5
In recent years, there have been several reported cases of PL developing after vaccination. Here, we present a case of PLC that followed the influenza vaccine, and a revision of cases of PL associated with vaccination.
A 66-year-old man with a past medical history of type 2 diabetes and hypertension presented to our Dermatology Department with a pruriginous rash that had started three days after he was vaccinated against the influenza virus. The lesions first appeared on the forearms and later spread to the trunk and lower limbs.
The patient worked as a patient care technician at a hospital, there was no history of recent infection or any other illness, and there were no other newly introduced systemic or topical drugs other than the vaccine.
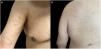
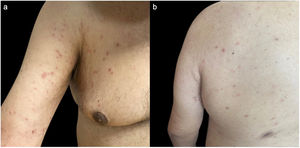
Clinical examination revealed multiple discrete erythematous papules with a fine silvery scale, located on the extensor aspects of the upper limbs, trunk and anterior aspect of the thighs (Fig. 1). The remaining physical examination was unremarkable.
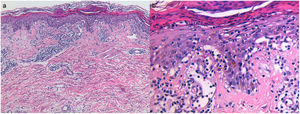
A punch biopsy of an abdominal papule was obtained and histopathological examination revealed hydropic degeneration of the basal cell layer, pityriasiform spongiosis, lymphocyte exocytosis, and isolated apoptotic keratinocytes. The corneal layer showed parakeratosis and neutrophiles (Fig. 2).
A diagnosis of PLC was made and the patient was treated with topical betamethasone dipropionate b.i.d., and a six-week course of doxycycline 100mg id. There was gradual improvement of established lesions and no recurrence after one-year of follow-up.
We have found 17 published cases of PL developing after vaccination. Most patients were male, aged under 40 years, with an average time between inoculation and the beginning of symptoms of 10 days. While PLEVA was the most commonly reported diagnosis, PLC was found in four cases. These findings can be found in Supplementary Table 1.
Local injection site reactions are the most commonly reported cutaneous adverse effects of the influenza vaccine, but there are reports of other reactions such as lichen planus6 and cutaneous leukocytoclastic vasculitis7 developing after this immunization. In this review, we identified only a case of PLEVA associated with the influenza vaccine.8
The pathophysiology of PL is incompletely understood. It is regarded by some authors as an inflammatory response to a wide range of external agents, such as infectious agents (Epstein–Barr and the human immunodeficiency virus for example)2,3 in PLEVA, and drugs (chemotherapy agents, hormonal therapy)8 in PLC. Other pathophysiologic explanations for PLC include T-cell dyscrasia (as progression to mycosis fungoides has been reported) and immune complex mediated hypersensitivity vasculitis.3
We believe this clinical case is related to the influenza vaccine due to temporal association between the vaccination and the symptoms, lack of other identifiable initiating factors, and lack of recurrence during follow-up.
Regarding treatment, it is noteworthy that clinical trials are lacking, making, sometimes, PL management a clinical challenge. Topical corticosteroids are often the first-line therapy, but complete responses are rare. Systemic antibiotics (erythromycin, doxycycline, minocycline, azithromycin) have a higher response rate and are usually well-tolerated.3,5,8 Various modalities of UV phototherapy have been used with good clinical response, being narrow-band UVB the most used due to its relative safety.5
In those cases where PLC seems to be associated with vaccination, we believe that the decision to receive further inoculations (when applicable) should be individualized, taking in consideration the risks associated with disease recurrence and the benefits of immunization. In this particular case, the patient repeated the inoculation the following year given his age, past medical history and occupation. There was no recurrence or other adverse effects.
We believe this case is of interest as PL following vaccination is relatively uncommon, and there is only one other reported case associated with the influenza vaccine. Follow-up for over one year and after a second immunization is also significant, since lack of published data of recurrence after a booster makes the decision of a new inoculation difficult.
Conflict of interestThe authors declare they have no conflict of interest.