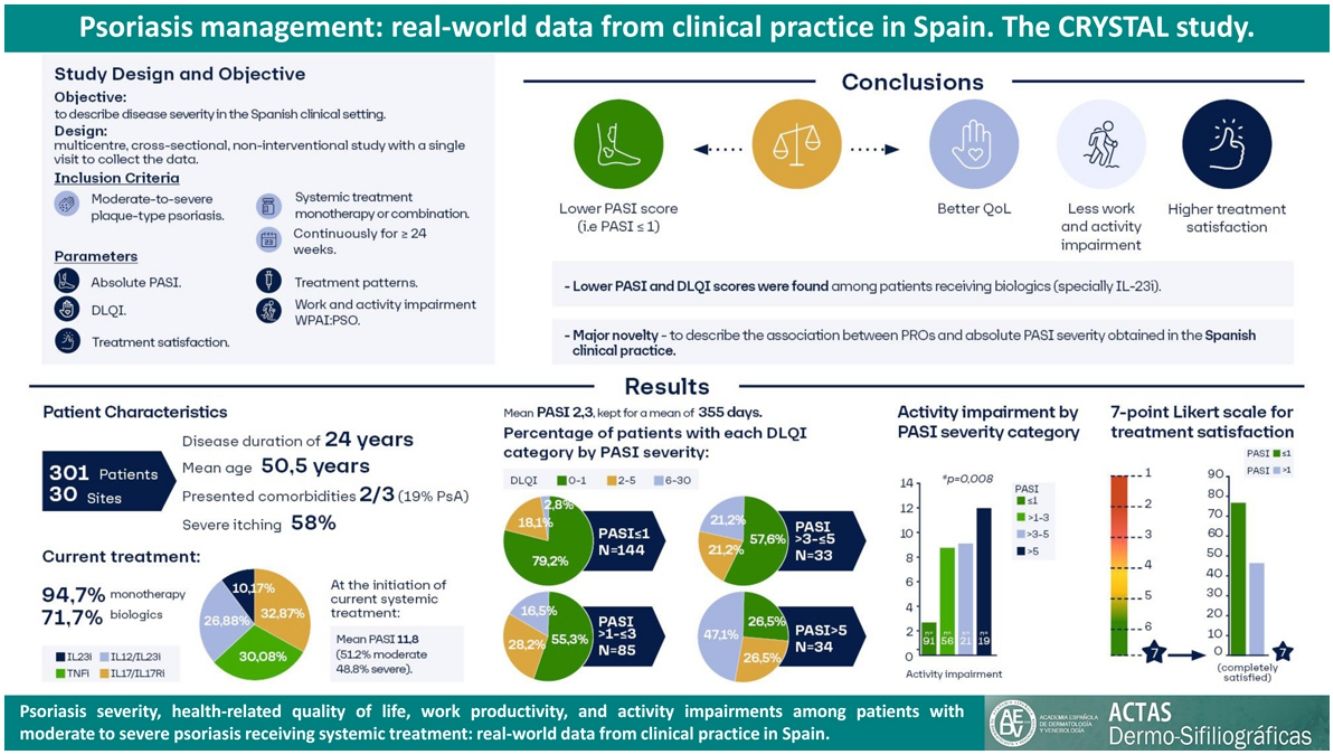
The treatment of psoriasis should not only focus on skin affectations but also weigh the parameters for health-related quality of life (HRQoL), thereby tackling the concept of cumulative life course impairment (CLCI) and treating the patient from a holistic perspective. The CRYSTAL study aimed to characterize psoriasis with real-word data from Spanish clinical practice in patients with moderate to severe disease who received continuous systemic treatment for at least 24 weeks by using the absolute Psoriasis Area and Severity Index (PASI) score and its correlation to HRQoL.
Material and methodsThis was a non-interventional, cross-sectional study conducted in 30 centers in Spain, with 301 patients between the ages of 18 and 75 years. The study collected data regarding current treatment and absolute PASI and their relationship to HRQoL using the Dermatology Life Quality Index (DLQI), to activity impairment using the Work Productivity and Activity Impairment (WPAI) questionnaire, and to treatment satisfaction.
ResultsThe mean (SD) age was 50.5 (12.5) years, with a duration of disease of 14 (14.1) years. The mean (SD) absolute PASI reported was 2.3 (3.5), with 28.7% of patients presenting with PASI from >1 to ≤3 and 22.6% with PASI>3. Higher PASI scores were associated with higher DLQI (p<0.001) and WPAI scores and lower levels of treatment satisfaction (p<0.001).
ConclusionsThese data indicate that achieving lower absolute PASI values may correlate not only with better HRQoL but also with better work productivity and treatment satisfaction.
El tratamiento de la psoriasis debe centrarse más allá de las afectaciones cutáneas, y valorar la calidad de vida relacionada con la salud (CVRS), abordando así el concepto de discapacidad acumulada en el transcurso vital y tratando al paciente desde una perspectiva holística. El estudio CRYSTAL tuvo como objetivo caracterizar la psoriasis con datos de la práctica clínica española en pacientes con enfermedad moderada a grave que recibieron tratamiento sistémico continuado durante al menos 24 semanas mediante la puntuación absoluta del Índice de la Severidad del área de Psoriasis (PASI) (medida del estado de la enfermedad en un momento dado y no por comparación con una puntuación basal) y su correlación con la CVRS.
Material y métodosSe trata de un estudio no intervencionista, transversal, realizado en 30 centros de España, con 301 pacientes de edades comprendidas entre los 18 y los 75 años. Se recogieron datos relativos al tratamiento actual y al PASI absoluto y su relación con la CVRS mediante el Índice de Calidad de Vida en Dermatología (DLQI), con el deterioro de la actividad mediante el Cuestionario para el Deterioro de la Actividad y la Productividad Laboral (WPAI) y con la satisfacción con el tratamiento.
ResultadosLa edad media (DE) fue de 50,5 (12,5) años, con una duración de la enfermedad de 14 (14,1) años. La media (DE) del PASI absoluto notificado fue de 2,3 (3,5), con 28,7% de pacientes que presentaban un PASI de > 1 a ≤ 3 y 22,6% con un PASI > 3. Las puntuaciones más altas del PASI se asociaron a puntuaciones más altas del DLQI (p < 0,001) y del WPAI y a niveles más bajos de satisfacción con el tratamiento (p < 0,001).
ConclusionesEstos datos indican que alcanzar valores absolutos más bajos de PASI puede correlacionarse no solo con una mejor CVRS, sino también con una mejor productividad laboral y satisfacción con el tratamiento.
Psoriasis is a chronic inflammatory skin disease with a prevalence in Spain of approximately 2.69%.1 The disease and the comorbidities associated affect patients’ health-related quality of life (HRQoL) and work and non-work related activities.2 Detriments of HRQoL in these patients are comparable to other major chronic diseases,3 and the chronic nature of psoriasis has an ongoing effect leading to a cumulative life course impairment (known as CLCI), where the burden of stigma, physical and psychological comorbidities, coping strategies and external factors interact and obstacle patients reaching their full life potential.4,5
Moderate to severe psoriasis is treated with systemic treatments, including conventional agents (methotrexate, cyclosporine, acitretin, and fumaric acids), phototherapy, biologic agents (tumor necrosis factor-α inhibitors [TNFi], interleukin (IL)-12/23 inhibitors [IL-12/23i], IL-17/IL-17 receptor inhibitors [IL-17i/IL-17Ri], and IL-23 inhibitors [IL-23i]), and an oral small molecule that inhibits phosphodiesterase-4 (apremilast). The achievement of optimal therapeutic targets has increased with the approval of new treatment options.6
The Psoriasis Area and Severity Index (PASI) and the Dermatology Life Quality Index (DLQI) are used to assess the effectiveness and changes in the quality of life, respectively, of systemic therapy.7 PASI can be measured as a relative or absolute score, with the absolute score being used in clinical trials of newer biologics8,9 and increasingly in clinical practice. According to the consensus of the Spanish Psoriasis Group, an absolute PASI≤3 is defined as an adequate therapeutic target, and an absolute PASI equal to zero as an optimal therapeutic target.8 The absolute PASI score is also included in the decision algorithm for psoriasis treatment in the French and Italian guidelines.10,11
There are correlations between PASI and DLQI,12 which depend on type of treatment, localization of the lesions,13 and use of relative versus absolute PASI.8 The assessment of HRQoL and work productivity based on disease severity and the wide range of systemic treatments remains an unmet need. A nationwide psoriasis patient registry in Spain (Biobadaderm14,15) collects safety and effectiveness data, but it does not collect data on HRQoL, work productivity, or patient satisfaction. The main objective of the present study was to describe psoriasis severity in patients with moderate to severe psoriasis receiving systemic treatment in the Spanish clinical setting using absolute PASI scores. In addition, we aimed to describe treatment patterns, HRQoL, work and activity impairment, and treatment satisfaction in these patients.
Materials and methodsCRYSTAL was a multicenter, cross-sectional, non-interventional study conducted between May and December 2020 at 30 medical centers in Spain. A single visit collected the informed consent and data, and all treatments were administered according to routine clinical practice. The study was approved by the CEIm Hospital Universitario La Princesa and conducted in accordance with the Declaration of Helsinki, the guidelines for Good Pharmacoepidemiology Practices of the International Society for Pharmacoepidemiology, and the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.
This study included patients who consented to participate, eligible with age between 18 and 75 years old; confirmed diagnosis of moderate to severe chronic plaque-type psoriasis; had received any approved systemic treatment for psoriasis, either as monotherapy or combination therapy, continuously for at least 24 weeks; had absolute PASI assessed at the start of their current systemic treatment (including a window from 30 days before to 7 days after); and had an absolute PASI assessment at the study visit. Patients who were currently or had previously received treatment with any investigational intervention within 1 month or 5 half-lives of the agent were not eligible.
The collection of current data at the study visit included sociodemographic and anthropometric characteristics; smoking habits; disease severity by absolute PASI; clinical characteristics; comorbidities; treatment for psoriasis; and patient-reported outcomes (PROs) in terms of HRQoL (DLQI), activity impairment (Work Productivity and Activity Impairment [WPAI] questionnaire), and patient satisfaction with treatment. Retrospective data collection included disease characteristics at psoriasis diagnosis, clinically relevant medical history, past treatments for psoriasis, and information about the current treatment from its initiation until enrollment (i.e., date of initiation, starting dosage, and dosage intensifications). The validated versions of the DLQI16 and the WPAI for psoriasis (WPAI:PSO)17 questionnaires for Spain were used with permission. Treatment satisfaction was measured using a single-item 7-point Likert scale, ranging from 1 (completely dissatisfied) to 7 (completely satisfied).
For the statistical analysis, a sample size of 300 patients was determined to provide estimation precisions (defined as the width of the 95% confidence interval [CI] of the mean) of ±0.11 for the absolute PASI scores, based on previous clinical studies. Frequency counts and percentages were reported for all categorical variables. Means and standard deviations (SD) were reported for all continuous variables. Mann–Whitney, Kruskal–Wallis, Chi-square, and the Fisher exact test were used to determine differences between the study variables, based on their distribution. The Spearman test was used to analyze correlations between variables. A linear regression analysis assessed associations between absolute PASI at study visit and the following characteristics: sex; previous psoriasis treatments; number of previous treatment cycles; family history of psoriasis; current systemic treatment; age; body mass index (BMI); medical history; absolute PASI; psoriasis duration; history of psoriatic arthritis, spondylitis, enthesitis, dactylitis, and pruritus; and difficult-to-treat areas at current systemic treatment initiation. A multiple linear regression model was built using characteristics with p<0.20 in bivariate analyses. Missing data were not considered in the analyses. The statistical analyses were performed with IBM SPSS Statistics, version 22.0 (IBM Corp., Armonk, USA), with significance set at the p<0.05 level.
ResultsA total of 309 consecutive patients were screened for the study, with 301 included. The eight excluded patients were ineligible because they did not meet all the selection criteria.
Baseline patient demographic and clinical characteristics are presented in Table 1. The mean (SD) age was 50.5 (12.5) years, and most patients were men (62.1%). Almost two-thirds of the patients (63.8%) had at least one comorbidity, and 58 (19.3%) patients had psoriatic arthritis, which was active in 30 of them. Patients had a mean (SD) duration of disease of 24 (14.1) years; after 13.5 (11.8) years they received systemic therapy. At the start of the current systemic treatment, patients had a mean absolute PASI of 11.8 (6.8), where 51.2% had moderate and 48.8% severe psoriasis. Severe pruritus was reported by 57.8% of patients at the beginning of the treatment and by 27.9% at the study's conclusion (Supplementary Table 1).
Baseline demographics and clinical characteristics.
| Patients (n=301) | |
|---|---|
| Age, years, mean (SD) | 50.5 (12.5) |
| Sex, male, n (%) | 187 (62.1) |
| Weight, kg, mean (SD) | 82.6 (17.1) |
| BMI, kg/m2, mean (SD) | 28.8 (5.5) |
| Smoker, n (%) | 100 (33.2) |
| Age at symptoms initiation, years, mean (SD) | 26.3 (13.9) |
| Age at psoriasis diagnosis, years, mean (SD) | 27.8 (14.1) |
| Psoriasis duration, years, mean (SD) | 24 (14.1) |
| Psoriasis severity at diagnosis, n (%) | |
| Moderate | 118 (39.2) |
| Severe | 72 (23.9) |
| Family history of psoriasis, n (%) | 165 (54.8) |
| Comorbidities,an (%) | 192 (63.8) |
| Arterial hypertension | 70 (23.3) |
| Dyslipidemia | 55 (18.3) |
| Depression/anxiety | 31 (10.3) |
| Diabetes | 27 (9.0) |
| Non-alcoholic fatty liver | 23 (7.6) |
| History of psoriatic arthritis, diagnosed or suspected | 58 (19.3) |
| Active, n (%) | 30 (51.7) |
| Age at symptoms initiation, years, mean (SD) | 39.4 (12.6) |
| Age at systemic therapy initiation, years, mean (SD) | 41.3 (13.3) |
| Time from psoriasis diagnose to systemic therapy initiation, years, mean (SD) | 13.5 (11.8) |
| Moderate | 13.2 (12.9) |
| Severe | 13.1 (10.9) |
| Disease duration at current systemic therapy initiation, years, mean (SD) | 20.4 (13.8) |
| PASI at current systemic therapy initiation, mean (SD) | 11.8 (6.8) |
At the initiation of current systemic treatment, 95.3% of patients (n=287) had received previous treatment (65.4% topical and 85.7% systemic). The regimen was monotherapy in most cases (88.8%), and the types of treatment received were conventional agents (87.2%, of which 75.6% was methotrexate), phototherapy (41.1%), biologic agents (40.7%, of which 73.3% was TNFi), apremilast (2.3%), and fumaric acid (2.3%).
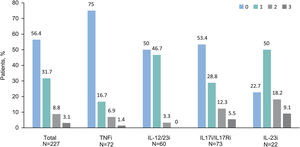
At the study visit, 94.7% (n=285) of patients were treated with monotherapy: 71.7% (n=213) with a biologic agent (23.6% [n=71] with IL-17i/IL-17Ri, 21.6% [n=65] with TNFi, 19.3% [n=58] with IL-12/23i, and 7.3% [n=227.3%] with IL-23i), 12.6% (n=38) with methotrexate, 7.0% (n=21) with apremilast, and 3.3% (n=8) with other agent. Other patients (5.3% [n=16]) received a combination of systemic treatments. Among patients with biologics, 56.4% (n=128) had not received a previous biologic treatment before their current treatment, 31.7% had received one, 8.8% had received two, and 3.1% had received three (Fig. 1). Biologic treatment was intensified (i.e., dose increased or time between doses decreased) in 17.2% (n=39). When each biologic treatment was considered, intensifications were observed in patients receiving IL-12/23i (31.7%), followed by TNFi (13.9%), IL-17i/IL-17Ri (13.7%), and IL-23i (4.5%).
Number of previously received biologics by current biologic treatment. Patients are grouped by biologic treatment received at study visit. Bars indicate percentage of patients treated with biologic agents at study visit who previously received up to 3 biologics agents (n=227). Abbreviations: IL, interleukin; IL-12/23i, IL-12/23 inhibitors; IL-17i, IL-17 inhibitors; IL-23i, IL-23 inhibitors; TNFi, tumor necrosis factor-α inhibitors.
At study visit, 50.8% (n=151) of patients were treated with concomitant topical therapy, mainly a corticosteroid (64.2%). Among patients treated with biologics, 62 (20.9%) had concomitant topical therapy and 151 (50.8%) had not.
At study visit, the mean (SD) absolute PASI was 2.3 (3.5) (n=296), maintained for a mean (SD) time of 355 (648) days (n=191). 48.6% had PASI≤1, 28.7% had PASI>1–3, and 22.6% had PASI>3). More than one third of patients had a PASI>2 (35.1%). Patients treated with biologics had the lowest PASI score; PASI≤1 was achieved by 68.2% of patients with IL-23i, 58.6% with IL-17i/IL-17Ri, and 53.1% with TNFi (Table 2).
PASI severity by systemic treatment.
| Treatment | n | Absolute PASI (n=296) | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | ≤1, n (%) | >1–3, n (%) | >3–5, n (%) | >5, n (%) | ||
| Monotherapy | 281 | 2.3 (3.3) | 137 (48.8) | 82 (29.2) | 32 (11.4) | 30 (10.7) |
| Methotrexate | 37 | 3.4 (4.1) | 12 (32.4) | 15 (40.5) | 3 (8.1) | 7 (18.9) |
| Apremilast | 21 | 3.6 (4.4) | 2 (9.5) | 11 (52.4) | 7 (33.3) | 1 (4.8) |
| IL-12/23i | 57 | 2.3 (3.3) | 28 (49.1) | 16 (28.1) | 6 (10.5) | 7 (12.3) |
| IL-17i/IL-17Ri | 70 | 1.7 (2.4) | 41 (58.6) | 15 (21.4) | 7 (10.0) | 7 (10.0) |
| IL-23i | 22 | 1.2 (2.6) | 15 (68.2) | 5 (22.7) | 1 (4.5) | 1 (4.5) |
| TNFi | 64 | 1.9 (3.0) | 34 (53.1) | 19 (29.7) | 5 (7.8) | 6 (9.4) |
| Othera | 10 | 3.4 (5.3) | 5 (50.0) | 1 (10.0) | 3 (30.0) | 1 (10.0) |
| Combination therapyb | 15 | 4.0 (5.3) | 7 (46.7) | 3 (20.0) | 1 (6.7) | 4 (26.7) |
| Total | 296 | 2.3 (3.5) | 144 (48.6) | 85 (28.7) | 33 (11.1) | 34 (11.5) |
Combination therapy includes acitretin+phototherapy; methotrexate+apremilast; methotrexate+IL-12/23i; methotrexate+IL-17i/IL-17Ri; methotrexate+TNFi; methotrexate+acitretin+apremilast; salazopyrin+TNFi.
Abbreviations: IL, interleukin; IL-12/23i, IL-12/23 inhibitors; IL-17i/IL-17Ri, IL-17/IL-17 receptor inhibitors; IL-23i, IL-23 inhibitors; PASI, Psoriasis Area and Severity Index; SD, standard deviation; TNFi, tumor necrosis factor-α inhibitors.
The multiple linear regression model revealed that conventional systemic treatment (estimate, 1.221 [95% CI, 0.33–2.11; p=0.007]) and apremilast (estimate, 1.883 [95% CI, 0.46–3.31; p=0.010) were associated with higher PASI scores at study visit (Supplementary Table 2).
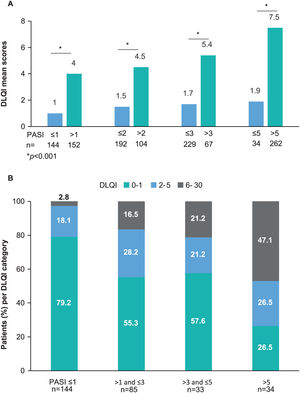
Impairments in HRQoL (DLQI scores≥2) were observed in 36.5% (n=110) of patients. The mean (SD) DLQI score at study visit was 2.5 (4.4). DLQI scores increased with higher PASI scores (p<0.001) (Fig. 2); DLQI scores ranged from 1.0 in patients with PASI≤1–7.5 in patients with PASI>5. DLQI scores between 0 and 1 (no effect) were observed in most patients with PASI≤1 (79.2%) and in only 26.5% of patients with PASI>5. Among those receiving biologics, only patients receiving IL-23i had a mean score lower than 1 (0.8) (Table 3).
DLQI score by PASI severity. (A) DLQI mean scores by PASI severity category, *p value<0.001 (Mann–Whitney's test) and (B) Percentage of patients with each DLQI category (0–1: no effect; 2–5: small effect; 6–30: moderate/very large/extremely large effect) by PASI severity category, with the variables compared using the hypothesis test χ2 (Chi-squared), with p value<0.001 (N=301).
DLQI score by systemic treatment.
| Treatment | n | DLQI score (n=301) | |||
|---|---|---|---|---|---|
| Mean (SD) | 0–1, n (%) | 2–5, n (%) | 6–30, n (%) | ||
| Monotherapy | 285 | 2.4 (4.3) | 184 (64.6) | 65 (22.8) | 36 (12.6) |
| Methotrexate | 38 | 2.9 (4.6) | 22 (57.9) | 9 (23.7) | 7 (18.4) |
| Apremilast | 21 | 3.2 (3.7) | 9 (42.9) | 7 (33.3) | 5 (23.8) |
| IL-12/IL-23i | 58 | 2.8 (4.7) | 37 (63.8) | 13 (22.4) | 8 (13.8) |
| IL-17i/IL-17Ri | 71 | 1.9 (3.7) | 46 (64.8) | 20 (28.2) | 5 (7.0) |
| IL-23i | 22 | 0.8 (1.6) | 19 (86.4) | 2 (9.1) | 1 (4.5) |
| TNFi | 65 | 2.6 (4.9) | 44 (67.7) | 13 (20.0) | 8 (12.3) |
| Othera | 10 | 3.2 (5.4) | 7 (70.0) | 1 (10.0) | 2 (20.0) |
| Combination therapyb | 16 | 4.4 (4.9) | 7 (43.8) | 4 (25.0) | 5 (31.2) |
| Total | 301 | 2.5 (4.4) | 191 (63.5) | 69 (22.9) | 41 (13.6) |
Combination therapy includes acitretin+phototherapy; methotrexate+apremilast; methotrexate+IL-12/23i; methotrexate+IL-17i/IL-17Ri; methotrexate+TNFi; methotrexate+acitretin+apremilast; salazopyrin+TNFi.
Abbreviations: DLQI, Dermatology Life Quality Index; IL, interleukin; IL-12/23i, IL-12/23 inhibitors; IL-17i/IL-17Ri, IL-17/IL-17 receptor inhibitors; IL-23i, IL-23 inhibitors; SD, standard deviation; TNFi, tumor necrosis factor-α inhibitors.
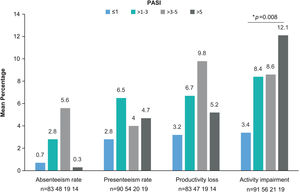
The WPAI questionnaire was completed by 299 patients. Employed patients (n=191, 63.9%) worked a mean (SD) of 34.8 (17)h weekly and reported a 1.8% absenteeism rate (work time missed), 4.2% presenteeism rate (reduced effectiveness while working), 5.1% productivity loss (overall work impairment), and 7.4% impairment in non-work-related activities (Table 4). Statistically significant differences were observed for activity impairment (p=0.008), with patients with PASI≤1 showing the lowest activity impairment rate (Fig. 3). For presenteeism, patients with PASI≤2 had lower rates (2.9%) than patients with PASI>2 (6.9%; p=0.034).
WPAI domains by systemic treatment.
| WPAI, mean (SD) %, [n] | ||||
|---|---|---|---|---|
| Treatment | Absenteeism | Presenteeism | Productivity loss | Activity impairment |
| Monotherapy | 1.3 (8.3) [158] | 3.9 (12.6) [174] | 4.1 (13.2) [151] | 6.8 (17.4) [283] |
| Methotrexate | 6.8 (23.4) [18] | 8.5 (19.8) [20] | 9.5 (22.3) [17] | 10.0 (22.7) [38] |
| Apremilast | 0 [8] | 6.2 (9.2) [8] | 6.2 (9.2) [8] | 8.0 (13.2) [20] |
| IL-12/23i | 0.5 (1.6) [35] | 1.1 (3.1) [37] | 1.3 (3.6) [35] | 6.7 (16.3) [58] |
| IL-17i/IL-17Ri | 0.5 (2.6) [42] | 2.4 (7.1) [46] | 2.4 (6.5) [42] | 5.9 (15.6) [70] |
| IL-23i | 0 [13] | 0 [13] | 0 [13] | 3.2 (14.9) [22] |
| TNFi | 0.8 (3.1) [35] | 7.1 (19.2) [42] | 7.7 (20.6) [35] | 6.8 (18.2) [65] |
| Othera | 2.0 (5.4) [7] | 0 (0) [8] | 2.0 (5.4) [7] | 7.0 (22.1) [10] |
| Combination therapyb | 10.0 (31.6) [10] | 8.5 (14.1) [13] | 21 (31.4) [10] | 18.1 (25.6) [16] |
| Total | 1.8 (11.1) [168] | 4.2 (12.7) [187] | 5.1 (15.3) [167] | 7.4 (18.0) [299] |
Combination therapy includes acitretin+phototherapy; methotrexate+apremilast; methotrexate+IL-12/23i; methotrexate+IL-17i/IL-17Ri; methotrexate+TNFi; methotrexate+acitretin+apremilast; salazopyrin+TNFi.
Abbreviations: IL, interleukin; IL-12/23i, IL-12/23 inhibitors; IL-17i/IL-17Ri, IL-17/IL-17 receptor inhibitors; IL-23i, IL-23 inhibitors; SD, standard deviation; TNFi, tumor necrosis factor-α inhibitors; WPAI, Work Productivity and Activity Impairment.
WPAI domains by PASI severity. Mean percentage of absenteeism (work time missed), presenteeism (reduced on-the-job effectiveness while working), productivity loss (overall work impairment), and daily activity impairment due to psoriasis (non-work-related activities) by PASI severity category. *p=0.008 (Kruskal–Wallis’ test). Abbreviations: PASI, Psoriasis Area and Severity Index; TNFi, tumor necrosis factor-α inhibitors; WPAI, Work Productivity and Activity Impairment.
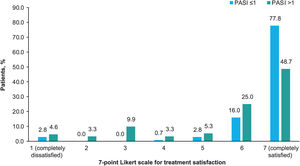
Treatment satisfaction was generally very high, with 62.8% of patients completely satisfied and 20.6% almost always satisfied. Based on the treatment received, more than half of the patients treated with biologics were completely satisfied (62.1% [n=36] treated with IL-12/23i; 69.0% [n=49] with IL-17i/IL-17Ri; 75.4% [n=49] with TNFi; and 95.5% [n=21] with IL-23i), and fewer than half of the patients treated with apremilast (33.3% [n=7]), methotrexate (44.7% [n=17]) and other agents (40% [n=4]) were completely satisfied. A higher treatment satisfaction was observed with a lower PASI (Chi-square test, p<0.001; Fig. 4).
Treatment satisfaction. Percentage of patients who gave each of the 7 possible responses to the question “How would you rate your level of satisfaction with the overall control of your psoriasis with your current treatment?” according to PASI severity. The options of the Likert scale were: 1 (completely dissatisfied), 2 (almost always dissatisfied), 3 (somewhat dissatisfied), 4 (neither satisfied nor dissatisfied), 5 (somewhat satisfied), 6 (almost always satisfied), 7 (completely satisfied). The patients were divided into 2 groups by PASI score (≤1 and >1). The variables were compared using the hypothesis Chi-square test, with p<0.001. Abbreviation: PASI, Psoriasis Area and Severity Index.
The CRYSTAL study, conducted in a cohort of 301 Spanish patients with moderate to severe chronic plaque-type psoriasis receiving continuous systemic treatment for at least 24 weeks, showed a mean absolute PASI score of 2.3 at the time of study. Increased disease severity was associated with a higher negative effect on HRQoL and activity impairment, as measured by DLQI and WPAI, respectively. The major novelty of the present study was to describe the association between PROs and absolute PASI severity obtained in the Spanish clinical practice.
Fifteen years ago in Spain, approximately 42% of patients with psoriasis (from a sample of 561) were treated with systemic therapy (traditional or biologics), and the mean PASI score was 9.6.18 The average PASI in our study is considerably lower than in the above-mentioned study, even though all patients had been diagnosed with moderate to severe psoriasis. This may suggest the management of psoriasis in Spain has improved, possibly owing to the wider availability of biologics, although, for the previous study, there was not the prerequisite of receiving systemic treatment for at least 24 weeks. In our study, lower PASI scores are found among patients receiving biologics than those receiving conventional treatments or apremilast. The lowest average PASI is observed in patients treated with IL-23i, followed by those receiving IL-17i/IL-17Ri. However, 35% of patients had an absolute PASI>2. These data indicates that there is still a long road ahead to reach the therapeutic goal of PASI≤2 in all patients, as recently proposed.9
The most prevalent comorbidities (arterial hypertension, dyslipidemia, depression/anxiety, and diabetes) were in line with previous observations in Spain.19,20 A prevalent comorbidity was psoriatic arthritis, diagnosed or suspected in almost 20% of patients approximately 11 years after diagnosis. Both the prevalence and time from diagnosis of psoriasis to the initiation of psoriasis arthritis symptoms was consistent with other studies.19–22 Cardiovascular risk factors and a high BMI have both been associated with vascular conditions and mortality in patients with psoriasis.23 The presence of these comorbidities and their implications for the overall health of the patient highlights the importance of screening for cardiovascular risk factors, psoriatic arthritis, and other comorbidities by dermatologists and for treating the patient in collaboration with other specialists.7,23
Most patients were treated with monotherapy, in line with the fact that there is no approved indication for any combination of a biologic with conventional systemic therapy in psoriasis. Moreover, 17.2% of patients had intensifications in their biologic treatment further than TNFi, confirming that off-label doses of biologic agents for psoriasis still occur in clinical practice,24 and, half of patients were treated with concomitant topical therapy. Considering all systemic therapies, patients treated with IL-23i required less concomitant topical therapy and fewer treatment intensifications, which could translate into cost savings for the healthcare system, decreasing the elevated cost of treating patients with more severe disease using biologics.25,26
We had observed a correlation between DLQI and PASI scores, mirroring previous studies,27,28 and indicating that PASI values≥3 have a considerable negative effect on patient quality of life, but PASI≤1 is even more pronounced in its positive effect on quality of life.
A 2020 study assessing the work productivity and economic consequences of psoriasis in six countries29 showed an annual loss of almost 4000 USD per patient in Spain due to indirect costs. Although our study did not assess the economic burden of psoriasis, it does show that the higher the PASI score, the higher the activity impairment and consequent economic burden.
The level of satisfaction that patients with psoriasis find with their treatment dictates their level of adherence to it30 and that level of satisfaction correlates with absolute PASI scores. Patients with higher PASI values are less satisfied with their current treatment. To our knowledge, this is the first time this type of correlation has been reported in psoriasis.
These results show the benefits of achieving higher treatment targets for patients in many aspects of their daily lives such as perceived quality of life. The impact of an adequate control of psoriasis on the concept of cumulative life course impairment merits future research.
This study has limitations. First, it includes large hospitals of national reference and smaller regional ones, with heterogeneous management of the disease, which may impede a more realistic sampling for patients with psoriasis. Second, although the sample size is adequate, for some types of analysis there is little representation, which may prevent reaching solid conclusions. Lastly, the study was carried out during the COVID-19 pandemic, and, therefore, some patient monitoring had to be done by phone.
ConclusionsThis study reveals an improvement in the management of psoriasis in the Spanish clinical setting in the last years, with a mean PASI score of 2.3. Patients receiving biologics had lower PASI and DLQI scores, especially those receiving IL-23i. Patients with low versus high absolute PASI values present with a better quality of life, higher satisfaction with treatment, and less work activity impairment. Therefore, for the benefit of patients with psoriasis, lower absolute PASI values (including PASI≤1) should be the goal.
Conflict of interestFinancial support for the study was provided by AbbVie. AbbVie participated in the study design and conduct, interpretation of data, review, and approval of the publication. No honoraria or payments were made for authorship.
ED has participated as an advisory board member and consultant; received grants, research support, and honorarium for speaking; and/or participated in clinical trials for AbbVie/Abbott, Almirall, Amgen, Celgene, Janssen-Cilag, Leo Pharma, Lilly, MSD/Schering-Plough, Novartis, Pfizer, and UCB. DV has participated in clinical trials and/or received honoraria as a consultant, investigator, or speaker for AbbVie, Janssen, Lilly, Novartis, and UCB. AR has participated in clinical trials and/or received honoraria as a consultant, investigator, or speaker for AbbVie, Almirall, Amgen, Janssen, Leo Pharma, Lilly, Novartis, and UCB. RR has participated on advisory boards, as a speaker, and/or in clinical trials for AbbVie, Almirall, Boehringer Ingelheim, Celgene, Janssen, Leo Pharma, Lilly, MSD, Novartis, Pfizer, UCB. JM has participated as speaker and as investigator in clinical studies with AbbVie, Janssen, Leo Pharma, Lilly, and Novartis. MJO has participated as consultant and/or speaker for AbbVie, Amgen, Leo Pharma, and Novartis. CV has participated as consultant and/or speaker for AbbVie, Janssen, Leo Pharma, Novartis. PI and NC are employees of AbbVie and may own AbbVie Stock. MTB, EZ and LM have no conflict of interest. AZ has participated as consultant, speaker, and investigator in clinical trials with AbbVie, Almirall, Amgen, Biogen, Celgene, Janssen, Leo Pharma, Lilly, Novartis, Pfizer, and Reig Jofré.
The authors thank Laura Prieto del Val and Vanessa Chiganças from Evidenze Health España S.L. for their support in the study design, data analysis, and writing of the manuscript. These services were funded by AbbVie.