Post-implantation erythema (PIE) is a benign cutaneous entity that has been scarcely reported and is associated with the insertion of a foreign body in contact with the adjacent skin, traditionally electronic and metallic devices.1–3 Its appearance on the breasts is uncommon, and no additional associated symptoms are usually present.4,5
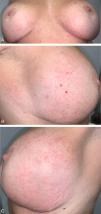
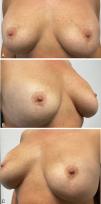
A 47-year-old woman, with no relevant past medical history, and a 4-month history poorly defined erythematous macules located symmetrically over the inferior and outer-lateral aspects of both breasts (Fig. 1A–C). Directed history taking revealed that she had undergone breast implant surgery 12 years earlier. The patient did not identify any other potential trigger and denied pruritus or other accompanying symptoms. Histologic examination showed a nonspecific inflammatory dermal infiltrate composed of lymphocytes and histiocytes with superficial perivascular distribution, without eosinophils, along with lymphatic vascular ectasia (Fig. 1D, E). No spongiosis or parakeratosis was observed. Patch testing—including the extended European baseline series, plastic and adhesive series, and silicone from breast implants—revealed no relevant findings. The lesions were treated with clobetasol propionate 0.5mg/g cream once daily, achieving complete resolution within two months (Fig. 2A–C).
(A–C) Initial clinical presentation: Diffuse erythematous macules located on the inferior and outer lateral regions of both breasts. Clinical images. (D) Superficial perivascular dermatitis with predominant lymphohistiocytic inflammatory infiltrate and lymphatic vascular ectasia with prominent endothelial cells (Hematoxylin–Eosin ×400). (E) Ectatic lymphatic vessels demonstrated by immunohistochemical staining (D2-40 ×200).
Until several years ago, these eruptions were classified under the term reticular telangiectatic erythema, without a clearly established pathogenesis. It was suggested that heat generated by electronic devices, together with the electromagnetic fields they produce, could contribute to local changes in the microvasculature adjacent to the implant, leading to erythema.1,2 Currently, the description of cases associated with non-electronic and non-metallic foreign bodies has led to the adoption of the broader term PIE. Our case supports a later etiologic theory proposing that lesions result from mechanical obstruction of local circulation due to pressure exerted by the foreign body on the skin.3–5 Clinically, PIE presents as asymptomatic erythematous macules that blanch with pressure, located in areas adjacent to the prosthetic material.4–6 It is a diagnosis of exclusion based on compatible clinical findings, histology showing a lymphohistiocytic inflammatory infiltrate with dermal vascular ectasia, negative patch testing, and a history of foreign-body implantation (with a temporal relationship that may extend several years after implantation).4,5,7
Table 1 illustrates the 12 published cases of PIE associated with non-electronic foreign bodies along with our own. PIE has been associated with knee, shoulder, and elbow joint prostheses; suture material; intrathecal drug delivery systems; hernia meshes; and breast implants. Analysis of the data shows a slight female predominance (7/13). Patient age ranged from 22 to 77 years, with most being older than 50 years (10/13). In 7 cases, PIE occurred within the first month after implantation, whereas in 3 cases it appeared>1 year later (2 associated with breast implants). The present case displays the longest latency period reported, appearing 12 years after implant placement. Histologically, dilation of dermal capillaries was observed in 7/13 cases, with lymphatic vessel ectasia specified in 2 reports. Sporadically, reactive dermal vascular proliferation and erythrocyte extravasation have been described. These findings further support the theory of compression and mechanical obstruction as a likely etiopathogenic mechanism. Various therapeutic approaches have been described. In 4 cases, a watch-and-wait strategy with close follow-up was adopted, with spontaneous resolution in 2 cases.1,4,5 Treatment with medium- to high-potency topical corticosteroids has also been used, achieving complete response in our patient. Pulsed dye laser therapy has been reported with good results after two sessions.4,5 Removal of the prosthetic material is generally not required. It was performed only in 4 cases in which device accessibility facilitated removal (3 involving drug-delivery systems and 1 related to non-absorbable suture material).8–10 All cases resolved completely, although only 2 reported the time to full resolution (2 weeks and 2 months).
Published clinical cases of post-implantation erythema associated with non-electronic devices.
| Author/Year | Age (years)/Sex | Implanted device (material) | Location | Time to onset after implantation | Cutaneous findings/associated symptoms | Histopathological features | Intervention performed | Implant removal | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Alegre-Sánchez et al., 20185 | 31/Female | Silicone breast implants | Breasts | 6 months | Erythematous patch with some associated telangiectasias/asymptomatic | Vascular dilation surrounded by mild lymphocytic infiltration | Two sessions of 595-nm pulsed dye laser | No | Marked clearing of erythema with no recurrence after 4-month follow-up |
| Segurado-Tostón et al., 20214 | 52/Female | Breast implants (Allergan silicone anatomical implants 410 MX 370g) | Breasts | 5 years | 2 erythematous plaques/asymptomatic | Mild superficial and mid-dermal perivascular lymphohistiocytic infiltrate with abundant vascularization | Expectant management and close follow-up | No | Not reported |
| Aneja et al., 20111 | 76/Female | Titanium elbow prosthesis | Left elbow | Days | Erythematous macules/asymptomatic | Mild fibrosis, inflammatory infiltrate, and telangiectatic blood vessels | Joint fluid aspiration | No | Resolution of lesions with intermittent course |
| Aneja et al., 20111 | 64/Female | Knee prosthesis (30% chrome-cobalt and 7% molybdenum) | Right knee | 7 months | 10-cm erythematous area with telangiectasias and mild edema/asymptomatic | Mild perivascular lymphocytic infiltrate with reactive vascular proliferation | Expectant management and close follow-up | No | Spontaneous resolution (time not specified) |
| Broekaert et al., 20123 | 77/Female | Shoulder hemi-prosthesis | Right shoulder | Several weeks | Diffuse reticular erythema/asymptomatic | Mild fibrosis with telangiectasias and dilated lymphatic vessels with perivascular lymphocytic infiltrate in the dermis | Expectant management and close follow-up | No | Spontaneous resolution after one year |
| Mercader-García et al., 20058 | 22/Male | Intrathecal drug delivery system | Left flank | 2 weeks | Ill-defined erythematous macules with peripheral telangiectasias/mild pruritus | Telangiectatic vessels in papillary and reticular dermis with perivascular lymphohistiocytic infiltrate | Device removal | Yes | Complete resolution two months after pump extraction |
| Milpied-Homsi et al., 20089 | 56/Male | Morphine pump (SynchroMed II, Medtronic) | Abdominal area | 3 weeks | Erythematous plaques/mild pruritus | Not described | Device removal | Yes | Not reported |
| Broekaert et al., 20123 | 66/Male | Drug delivery pump | Left flank | 1 week | Brown U-shaped reticular erythema/mild pruritus | Dermal dilated blood and lymphatic vessels with sparse perivascular lymphocytic infiltrate | Device removal | Yes | Resolution (time not specified) |
| Goeller et al., 20146 | 77/Male | Surgimesh XB polypropylene mesh (Aspide Medical, St. Etienne, France) | Mesogastrium | Days | Blanchable erythematous plaques/asymptomatic | Not described | IV antibiotics | No | Lesion persisted |
| Goeller et al., 20146 | 69/Male | Surgimesh XB polypropylene mesh (Aspide Medical, St. Etienne, France) | Mesogastrium | 14 months | Abdominal erythema/asymptomatic | Not described | Antibiotics | No | Lesion persisted |
| Goeller et al., 20146 | 70/Male | Surgimesh XB polypropylene mesh (Aspide Medical, St. Etienne, France) | Mesogastrium | 2 weeks | Erythematous patch/mild pruritus | Not described | Expectant management and close follow-up | No | Lesion persisted |
| Armengot-Carbo et al., 201610 | 61/Female | Non-absorbable suture thread | Left breast | 1 month | Erythema with reticular telangiectasias/asymptomatic | Dermal telangiectasias with intermediate lymphocytic infiltrate and epidermal atrophy | Material removal | Yes | Complete resolution 2 weeks after suture removal |
| Llamas-Segura et al. | 47/Female | Breast implant (material if known) | Breasts | 12 years | Symmetric erythematous macules | Perivascular lymphocytic infiltrate, vascular ectasia, and erythrocyte extravasation | Clobetasol propionate 0.5mg/g cream | No | Resolution at 2 months |
In conclusion, PIE is a recently described entity and represents a therapeutic challenge. We present the third reported case of PIE due to breast implants and compile all cases attributed to non-electronic devices, along with the therapeutic strategies employed. Recognition and further study of this entity will provide a solid foundation for advancing understanding of its still-unclear etiopathogenesis and optimal management.
Conflict of interestThe authors declare that they have no conflict of interest.
To María Narváez Simón, staff physician in the Department of Pathology at Hospital Universitario Clínico San Cecilio (Granada, Spain) for describing the histologic images of the case.