Adult dermatomyositis presents as a paraneoplastic syndrome in up to 25% of cases, but no clinical, histologic, or laboratory markers completely specific for paraneoplastic disease in dermatomyositis have been identified to date. Furthermore, studies on adult dermatomyositis do not usually report the frequency of cutaneous features of dermatomyositis in patients with associated cancer. Our aim was to review the characteristics of paraneoplastic dermatomyositis in patients seen at our hospital.
Material and methodsWe studied 12 cases of paraneoplastic dermatomyositis and recorded patient age and sex, associated cancer, time between onset of dermatomyositis and cancer, emergent cutaneous manifestations, muscle involvement, dysphagia, lung disease, and levels of creatine phosphokinase and circulating autoantibodies.
ResultsThe mean age of the patients was 61 years and the 2 most common malignancies were ovarian cancer and bladder cancer. The mean time between the diagnosis of cancer and dermatomyositis was 7 months and in most cases, the cancer was diagnosed first. Seven patients had amyopathic dermatomyositis. The most common cutaneous signs were a violaceous photodistributed rash sparing the interscapular area and a heliotrope rash, followed by Gottron papules and cuticle involvement. Superficial cutaneous necrosis was observed in 3 cases. Myositis-specific autoantibodies were not detected in any of the 6 patients who underwent this test.
ConclusionsParaneoplastic dermatomyositis is often amyopathic. There are no specific cutaneous markers for malignancy in dermatomyositis. Myositis-specific antibodies are not associated with paraneoplastic dermatomyositis.
La dermatomiositis del adulto es paraneoplásica hasta en una cuarta parte de los casos. Hasta la fecha no existe ningún rasgo clínico, histológico o analítico absolutamente específico de paraneoplasia en dermatomiositis. Además, los estudios sobre dermatomiositis del adulto no suelen hacer referencia a la frecuencia de aparición de los distintos signos cutáneos propios de la dermatomiositis en los casos asociados a cáncer. Por todo ello decidimos revisar nuestros casos de dermatomiositis paraneoplásicas.
Material y métodosEstudiamos 12 casos de dermatomiositis paraneoplásicas en los cuales se recogió la edad, el sexo, el cáncer asociado, el tiempo entre el inicio de la dermatomiositis y el cáncer, 9 signos cutáneos, afectación muscular, disfagia, enfermedad pulmonar, niveles de creatinfosfocinasa y de autoanticuerpos circulantes.
ResultadosLa media de edad fue de 61 años y los 2 cánceres asociados más frecuentes fueron el de ovario y el de vejiga. El tiempo medio transcurrido entre el diagnóstico del cáncer y el de la dermatomiositis fue de 7 meses, y en la mayoría el diagnóstico de cáncer precedió al de dermatomiositis. Siete pacientes tuvieron dermatomiositis amiopática. De los signos cutáneos, los más frecuentes fueron una erupción violácea fotodistribuida que respetaba la región interescapular y el rash heliotropo, seguidos de las pápulas de Gottron y la afectación de cutículas. Se encontró necrosis cutánea superficial en 3 casos. Los autoanticuerpos específicos de miositis resultaron negativos en los 6 casos estudiados.
ConclusionesLa dermatomiositis paraneoplásica es muchas veces amiopática. No existe ningún rasgo cutáneo específico de paraneoplasia en la dermatomiositis. Los anticuerpos específicos de miositis no se asocian a la dermatomiositis paraneoplásica.
Dermatomyositis, a rare inflammatory disease possibly of autoimmune origin, produces a characteristic skin rash and symmetrical proximal myopathy. Amyopathic dermatomyositis is a variant in which muscle involvement is absent or is very mild. The incidence of dermatomyositis shows a bimodal distribution, with 2 forms of presentation according to the age at onset of the disease and called respectively juvenile dermatomyositis and adult dermatomyositis. Juvenile dermatomyositis is not typically a paraneoplastic disease, and very few cases of juvenile dermatomyositis associated with cancer have been published.1 In fact, the frequency is so low that the association between juvenile dermatomyositis and cancer is considered anecdotal. In contrast, adult dermatomyositis is a paraneoplastic condition in 15% to 25% of cases.2–5 The pathogenic relationship between dermatomyositis and cancer is not fully understood.6 It would appear that the regenerating cells that appear in muscles with myositis express high levels of the specific antigens of myositis,7 and that these are the same as those expressed in various cancers associated with inflammatory myopathies. The link between cancer and dermatomyositis would thus appear to be the expression of antigens common to the cancer and to muscle tissue in some patients with dermatomyositis.
The first problem that we encountered on reviewing the literature on paraneoplastic dermatomyositis was that most of the studies published until relatively recent indistinctly included dermatomyositis and polymyositis, giving the risks of cancer for the two diseases together. However, it is ever more widely accepted that dermatomyositis and polymyositis are distinct entities that show differences not only in their pathogenic mechanisms8 but also in their epidemiology and risk of associated cancer.2 The results of many of those studies must therefore be interpreted with caution. Furthermore, the majority of the largest published series of paraneoplastic dermatomyositis were population-based series and did not accurately define the cutaneous manifestations.2,5,9,10 In view of this situation, in the present study we have reviewed those patients with paraneoplastic dermatomyositis diagnosed in our hospital, paying special attention to the description of the dermatologic manifestations.
Materials and MethodsWe performed a retrospective observational study of all patients diagnosed with paraneoplastic dermatomyositis in our hospital between January 1994 and January 2013. A total of 12 cases were included. The data sources for all the parameters studied were the medical histories of the patients, the biopsy library of the pathology department, and the image archive of our own department.
The following inclusion criteria were applied: presence of a skin rash that was clinically and histopathologically compatible with dermatomyositis, with or without the presence of a symmetrical proximal myopathy (defined as proximal weakness with or without elevation of the creatine kinase [CK]); and concomitant cancer (excluding basal cell carcinoma and cutaneous squamous cell carcinoma) first diagnosed or presenting relapse within a maximum of 2 years before the onset of the dermatomyositis. The characteristic skin rash was considered to be a violaceous rash in sun-exposed areas and/or a heliotrope rash and/or Gottron papules. The histopathology findings considered to be consistent with dermatomyositis were the presence of epidermal atrophy (except in the case of Gottron papules) with vacuolar degeneration of the basal layer, keratinocyte necrosis, and varying degrees of mucin deposition.
The following parameters were studied in each patient: age, sex, type of associated cancer, time between the onset of dermatomyositis and the cancer, skin rash (specifically, the presence or absence of violaceous poikiloderma in sun-exposed areas, cuticular dystrophy and telangiectasias, heliotrope erythema, Gottron papules, pruritus, poikiloderma of the scalp, flagellate erythema, erosions/ulcers, calcinosis), presence or absence of proximal symmetrical weakness of the extensor muscles, interstitial pulmonary fibrosis, dysphagia, CK levels, and circulating antibody levels (antinuclear antibodies [ANA], anti-DNA, anti-ribonucleoprotein [anti-RNP], anti-Jo1, anti-Mi1/Mi2, anti-Ro, anti-La, and anti-Sm).
ResultsTwelve cases of paraneoplastic dermatomyositis were included in the study. There were 6 men and 6 women aged between 43 and 84 years (mean age, 61 years). The clinical and laboratory characteristics of the 12 patients are shown in Table 1. The associated cancers were as follows: ovarian cancer in 3 patients, bladder cancer in 3 patients, 1 patient each with cancer of the lung, stomach, breast, prostate and cervix, and 1 patient with non-Hodgkin lymphoma. The time between the diagnosis of dermatomyositis and of the associated cancer (or its recurrence) varied between synchronic and a maximum of 20 months, with a mean of 7 months. Paraneoplastic dermatomyositis arose in the context of tumor recurrence in 4 patients. The diagnosis of paraneoplastic dermatomyositis was made after diagnosis of the cancer in all except 2 patients (cases 4 and8), in whom the cancer was detected in the cancer screening study performed because of the presence of dermatomyositis. Seven patients showed no muscle involvement and were thus diagnosed with amyopathic dermatomyositis. Muscle weakness was detected in 5 patients, 4 of whom had elevated CK levels. Three patients reported dysphagia. Only patient number 10 had interstitial lung disease.
Clinical and Laboratory Manifestations of the 12 Patients With Paraneoplastic Dermatomyositis.
| Case | Age, y | Sex | Malignancy | Interval,a mo | Myositis | Dysphagia | CK, U/L | Interstitial Lung Disease | Antibodies |
| 1 | 48 | M | Small cell lung cancer | 9 | Yes | No | 562 | No | ANA+ 1/40, anti-DNA–, anti Ro–, anti-La–, anti-RNP– |
| 2 | 74 | M | Bladder | 0 (recurrence) | No | No | Normal | No | ANA+ 1/160 |
| 3 | 68 | M | Bladder | 18 | Yes | No | 406 | No | ANA–, anti-Jo–, anti-Mi-1/Mi-2– |
| 4 | 58 | M | Gastric | 0 (at onset) | Yes | Yes | 5147 | No | Anti-Sm–, anti-DNA–, anti-Jo-1–, anti-RNP–, anti-Ro–, anti-La– |
| 5 | 43 | F | Infiltrating ductal breast cancer | 12 | Yes | Yes | 1399 | No | ANA+ 1/640, anti-Jo-1–, anti-RNP–, anti-Ro–, anti-La–, anti-Sm– |
| 6 | 84 | F | Diffuse large B-cell non-Hodgkin lymphomaBreast | 9 | No | No | NR | No | NR |
| 7 | 64 | M | Prostate | 0 | No | No | Normal | No | Anti-Jo-1–, ANA– |
| 8 | 61 | F | Ovary | 0 | Yes | Yes | NR | No | ANA– |
| 9 | 53 | F | Squamous cell cervical cancer | 20 (recurrence) | No | No | Normal | No | ANA–, anti-DNA– |
| 10 | 69 | M | Bladder | 15 (recurrence) | No | No | Normal | Yes | Anti-Jo-1–, ANA– |
| 11 | 55 | F | Ovary | 0 (recurrence) | No | NR | Normal | No | NR |
| 12 | 56 | F | Ovary | 3 | NR | NR | NR | No | NR |
| Total/mean | 61.08 | 6F/6M | 7.16 | 5 | 3 | 4 ↑ | 1 | 3 ANA+ |
Abbreviations: CK, creatine kinase; F, female; M, male; N, normal; NR, datum not recorded.
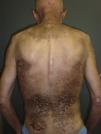
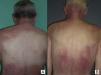
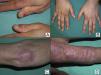
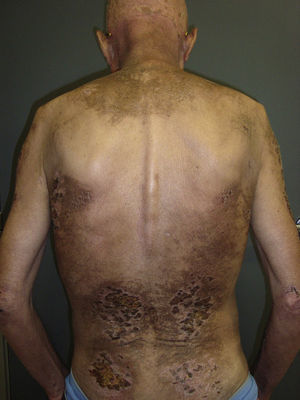
The most common features of skin involvement were poikiloderma in sun-exposed areas and heliotrope rash, present in 10 cases, followed by cuticlar alterations (dystrophia or periungual erythema) and Gottron papules, present in 9 patients. The central area of the back was spared in all patients, even in those with more extensive skin involvement. Less common findings were pruritus (5 cases), skin necrosis (3 cases) and, finally, flagellate erythema and poikiloderma of the scalp (2 cases each). Skin necrosis consisted predominantly of crusted, eroded plaques on the trunk, neck, or proximal regions of the limbs in all 3 cases. None of the patients presented calcinosis cutis. All the cutaneous manifestations are shown in Table 2.
Summary of the Skin Manifestations of the 12 Patients With Paraneoplastic Dermatomyositis.
| Case | Violaceous Rash in Sun-Exposed Areas | Cuticular Involvement | Heliotrope Erythema | Gottron Papules | Pruritus | Skin Necrosis | Flagellate Erythema | Poikiloderma of the Scalp | Calcinosis |
| 1 | + | + | + | + | + | + | NR | NR | No |
| 2 | + | NR | + | + | +++ | + | + | No | No |
| 3 | + | + | + | + | + | No | + | No | No |
| 4 | + | + | + | + | NR | +++ | NR | + | No |
| 5 | NR | + | +++ | + | NR | No | NR | NR | No |
| 6 | + | No | No | No | + | No | No | No | No |
| 7 | + | + | + | No | NR | No | No | + | No |
| 8 | + | + | ++ | + | + | No | NR | No | No |
| 9 | + | + | + | + | NR | No | NR | No | No |
| 10 | NR | + | + | NR | NR | No | No | No | No |
| 11 | + | + | + | + | NR | No | NR | NR | No |
| 12 | + | No | NR | + | NR | No | NR | NR | No |
| Total | 10 | 9 | 10 | 9 | 5 | 3 | 2 | 2 | 0 |
Abbreviations: +, symptom or sign present (++, marked; +++, severe); NR, datum not recorded.
Finally, 3 patients were positive for ANA (Table 1). The specific myositis antibody panel was negative in all 6 patients in whom it was performed (Table 1).
DiscussionA diagnosis of adult dermatomyositis compels us to exclude a possible paraneoplastic etiology. According to the largest series, 15% to 25% of cases of adult dermatomyositis are associated with cancer.2,5 This risk is present even in cases of amyopathic dermatomyositis.11–13 In our series, amyopathic dermatomyositis was detected in 7 of the 12 cases. The risk of associated cancer would appear to be higher in dermatomyositis (32%) than in polymyositis (14.9%).2
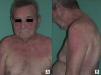
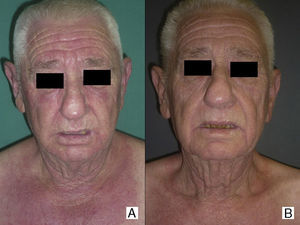
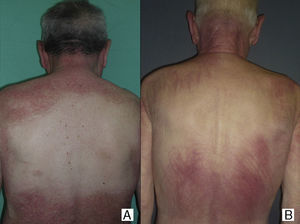
Paraneoplastic dermatomyositis can precede, coincide with, or develop after the diagnosis of cancer.14 In our series, the majority of cases were diagnosed after diagnosis of the cancer, either after the first manifestation of the cancer or as a result of its recurrence; in only 2 cases (patients 4 and8) was the cancer diagnosed as a result of the screening study performed because of the diagnosis of dermatomyositis. According to Callen et al.,14 the clinical course of paraneoplastic dermatomyositis may not always be linked to the underlying neoplasm. In agreement with reports from other authors,15–17 the clinical course of the dermatomyositis in our patients was clearly related to the course of the underlying cancer and, in a few patients, recurrence was detected earlier due to resurgence of the cutaneous manifestations rather than follow-up imaging studies. In addition, in those patients in whom anticancer treatment achieved a complete response, we observed spectacular improvements in both the cutaneous and the muscle manifestations of the dermatomyositis (Fig. 1).
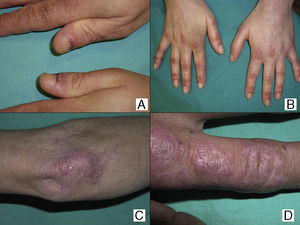
In cases of dermatomyositis that arise in patients previously diagnosed with cancer, the diagnosis of paraneoplasia is implicit in the diagnosis of the dermatomyositis, and screening studies are not indicated. However, in cases of adult dermatomyositis in patients with no previous diagnosis of cancer, the dermatologist is obliged to determine whether or not the patient has an associated neoplasm. Although many studies have looked for traits that would enable us to predict when dermatomyositis has a higher risk of being associated with cancer, little has been achieved.2,18 Of all the parameters studied, it would appear that those most consistently associated with a higher risk of cancer in patients with adult dermatomyositis are male gender and more advanced age.2 One clinical trait that has repeatedly been related with paraneoplastic dermatomyositis in the literature is skin necrosis.19–21 Skin necrosis is, in fact, almost the only purely dermatologic finding that has been considered to be associated with cancer in dermatomyositis, although no studies have conclusively demonstrated this relationship. In our series, skin necrosis was detected in 3 cases, and in all 3 the necrosis was relatively superficial and affected the skin of the trunk or of the proximal regions of the limbs in the form of crusted erosions, at least initially. In one of these patients (case1) we observed ulceration of Gottron papules on the elbow, and in another patient (case4) the skin necrosis became more widespread and deeper, even affecting the cuticles, though it was still most intense on the trunk and limbs and showed no apparent relationship with vasculitis (Fig. 2). We would like to draw particular attention to this point, as a variant of dermatomyositis associated with anti-MDA5 antibodies has recently been described22; this typically amyopathic form presents necrotic skin lesions and carries a low risk of associated cancer. In fact, dermatomyositis with anti-MDA5 antibodies is often associated with interstitial lung disease and skin ulcers are a habitual finding. However, the risk of cancer is very low, and the presence of these anti-MDA5 antibodies is probably predictive of a lower risk of malignancy in dermatomyositis, as only 1 of the 52 published cases of dermatomyositis associated with this antibody was paraneoplastic dermatomyositis.23 The skin necrosis described in dermatomyositis with anti-MDA5 antibodies typically affects the hands, the Gottron papules, and the elbows, and signs of vasculitis are observed on histology; this would suggest that the necrosis in this variant of dermatomyositis is completely different from the type of necrosis we observed in our patients with paraneoplastic dermatomyositis, in whom the necrosis was more superficial and showed a different distribution (trunk and proximal regions of the limbs). Apart from skin necrosis, no other cutaneous manifestations have been reported to be predictive of an association with malignancy except for the vesiculo-bullous variant of dermatomyositis.24 Vesiculo-bullous dermatomyositis is very rare, and reports of its association with malignancy are anecdotal. Isolated reports have also suggested that the presence of periungual erythema or the acute onset of cutaneous or muscle manifestations are predictive of a higher risk of cancer,4 though the majority of published studies do not support these hypotheses.
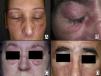
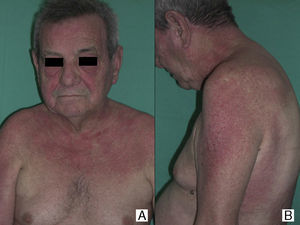
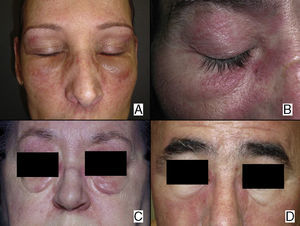
In our series, the most common presentation of paraneoplastic dermatomyositis was with an erythematous-violaceous rash, sometimes poikilodermic, in sun-exposed areas, preferentially affecting the face and also often the upper part of the trunk and the extensor surfaces of the upper limbs (Fig. 3). In some patients this rash can be more extensive, affecting a surface area similar to erythroderma. However, even in those cases, we observed that the central area of the mid back was systematically spared (Fig. 4). The presence of a palpebral or periocular rash, described as a heliotrope rash (Fig. 5, A-D), is also very common. In some patients this heliotrope rash consists mainly of intense erythema (Fig. 5B), but in the majority of our patients was associated with some degree of edema (Fig. 5D). In some cases the edema was marked and clearly dominated the picture over the erythema (Fig. 5, A andC). Gottron papules and cuticular involvement also appear to be quite common findings in paraneoplastic dermatomyositis, based on our results (Fig. 6). Of the other cutaneous manifestations observed in our patients (see Results), only the fact that calcinosis was not detected in any case merits particular mention. This is logical if we take into account that calcinosis is a feature more characteristic of juvenile dermatomyositis (not associated with cancer) than of adult dermatomyositis. Summarizing the cutaneous involvement in paraneoplastic dermatomyositis, we detected no findings that differed from those described for dermatomyositis in general. Because our study did not include a control group of adults with dermatomyositis not associated with cancer, we cannot state whether the prevalence of skin necrosis (25% in our series) was higher or lower than the prevalence in the population with non-paraneoplastic dermatomyositis. In a recent study of 39 cases of dermatomyositis, 11 of them paraneoplastic, the proportion of cases with skin necrosis was the same in the group associated with cancer and in the overall group.25 Another series of patients with dermatomyositis associated with anti-MDA5 antibodies23 also showed no relationship between skin necrosis and paraneoplasia, although those are unpublished data (personal report from Dr. David Fiorentino). Unfortunately, the largest studies of paraneoplastic dermatomyositis within the adult dermatomyositis population have not reported comparative data of the frequency of skin necrosis in the 2 groups of patients.2–4
Sparing of the central area of the back in patients no.2(A) and no.3(B). Flagellate erythema can be seen in both cases. (See also Fig. 2.)
Leaving skin manifestations to one side, there is another clinical trait that has been associated with a risk of cancer in various studies of adult dermatomyositis; this is the presence of interstitial lung disease. Lung involvement is considered to be a marker of a low risk of neoplasm in dermatomyositis.3,23 Of our patients, only one (case10) had interstitial lung disease. That patient had a history of bladder cancer 4 years before onset of the dermatomyositis and developed tumor recurrence 15 months after the skin manifestations appeared. The lung involvement was severe and, although it was detected in the tumor screening studies performed after making the diagnosis of dermatomyositis, it subsequently caused to pneumothorax with surgical emphysema. It is not known why dermatomyositis with interstitial lung disease is associated with a lower probability of cancer. It has been suggested that the prognosis of the lung disease is so poor that the short life expectancy of the patient leaves insufficient time for the cancer to be detected. However, this is just a theory that has not yet been confirmed.
Apart from the clinical manifestations, attempts have also been made to find laboratory markers that can predict a higher risk of cancer in paraneoplastic dermatomyositis. The most promising of these are the anti-155/140 antibodies.26,27 The presence of these antibodies in a patient with dermatomyositis has a high specificity and negative predictive value for the diagnosis of paraneoplasia. In contrast, several studies have also found the presence of antibodies traditionally associated with myositis, such as anti-Jo-1, anti-Mi-2, and anti-U1-RNP, to be associated with a low probability of cancer in dermatomyositis.28 It would appear that the combination of the 2 concepts—the absence of antibodies traditionally associated with myositis and the presence of anti-155/140 antibodies—provides a sensitivity and a negative predictive value of almost 100% in dermatomyositis.26 Among the patients in our study in whom these tests were performed, all showed an absence of the specific antibodies traditionally associated with myositis, which would support previous conclusions in the literature.
Anecdotally, it has also been reported that low levels of complement fraction C44 and elevated CK levels3 are associated with a higher risk of cancer, whereas lymphopenia is more likely to be associated with a lower risk of malignancy.4 However, these associations are much more controversial and have not been detected in the majority of published research. Only 4 of our patients presented elevated CK levels.
Despite all the above, when a diagnosis of adult dermatomyositis is made in daily clinical practice, skin necrosis is not usually present and, even if found, it has not been shown at a population level to imply a higher risk of cancer, as commented above. But, of course, its absence in no way excludes paraneoplasia. Anti-155/140 antibodies are still not usually available. Hence, for the time being, cancer screening must be performed in any case of adult dermatomyositis.
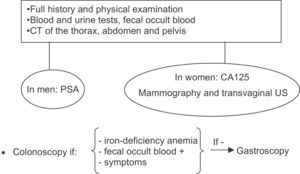
Cancer screening studies in adult dermatomyositis must be adapted to the tumor profile of the population to which the patient belongs. In Europe, the cancers associated with adult dermatomyositis include, in order of frequency, ovary, lung, breast, colon and rectum, stomach, and pancreas. Other associated cancers include prostate and non-Hodgkin lymphoma.2 By sex, the most common cancers associated with adult dermatomyositis in women are breast and ovary and, in men, are lung and colon and rectum. However, the risk profile is different in patients from southeast Asia, as the tumor most frequently associated with paraneoplastic dermatomyositis in that geographical region is nasopharyngeal cancer.5 A targeted history must be taken from all patients and a complete physical examination performed. Blood tests (including prostate specific antigen in men and CA125 in women), urinalysis, and fecal occult blood should also be performed. In women, mammography and transvaginal ultrasound are also recommended. In both sexes, the study is then completed with computed tomography (CT) of the thorax, abdomen, and pelvis.4 The use of more invasive investigations, such as colonoscopy, is reserved for patients over 50 years of age and those with suspicious symptoms, positive fecal occult blood, or iron-deficiency anemia. In any of these situations, if colonoscopy is normal, gastroscopy should be performed (Fig. 7). In patients from southeast Asia, however, the initial approach is to request study in otorhinolaryngology to exclude nasopharyngeal carcinoma. In a study with 55 patients with myositis (polymyositis and dermatomyositis), screening using conventional methods was compared with the use of positron emission tomography (PET)-CT, finding a similar efficacy.29 Those authors considered PET-CT to be more suitable for screening for dermatomyositis-related cancer because it avoided the need for a larger number of studies.
A negative result of targeted cancer screening studies in any patient should make us consider with what frequency the studies should be repeated. According to the literature, the highest risk of cancer occurs during the first year after diagnosis of dermatomyositis, and the risk then gradually decreases, although it never falls to the same level as in the general population. This appears to differ from the situation with polymyositis in which, after 5 years, the incidence of cancer equals that of the general population.2 In our series, most of the cases of cancer or of tumor recurrence occurred within 2 years after the diagnosis of dermatomyositis. The most widely accepted plan is to repeat the screening for cancer at least once a year for the first 3 years.
Regarding the management of paraneoplastic dermatomyositis, our aim must always be to control the underlying neoplasm. Patients in our series in whom tumor response was complete presented spectacular improvements or complete remission of the dermatomyositis. Furthermore, we detected recurrence of the dermatomyositis prior to tumor recurrence in several cases. When oncologic treatment is not possible or does not achieve a complete response, and in those cases in which anticancer therapy is delayed, the treatments most widely used to control the dermatomyositis are oral corticosteroids at a dose of 0.5-1mg/kg/d. If there is no adequate response or if the treatment has to be prolonged, methotrexate can be beneficial at doses between 7.5 and 15mg per week. In our series, a symptomatic response of the dermatomyositis was achieved with oral corticosteroids and/or methotrexate in all patients except one (patient8).
The main limitations of our study are its retrospective design, the number of patients included, and the absence of a control group formed of patients with nonparaneoplastic adult dermatomyositis. The absence of a control group meant that we could not draw meaningful conclusions on the potential value of certain skin manifestations in the identification of dermatomyositis as a paraneoplastic syndrome. However, with regard to the second limitation, many of the series looking for specific findings suggestive of malignancy in dermatomyositis have not included significantly larger numbers of cases associated with cancer.3,4,19,21,26 Finally, as this was a retrospective study, there was variability in the signs and symptoms recorded and in the additional tests performed.
Ethical DisclosuresProtection of human and animal subjects.The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they followed their hospital's regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Requena C, Alfaro A, Traves V, Nagore E, Llombart B, Serra C, et al. Dermatomiositis paraneoplásica: estudio de 12 casos. Actas Dermosifiliogr. 2014;105:675–682.