Although there is no universal consensus on the precise definition of “overlap” between psoriasis and atopic dermatitis, it is generally understood as the coexistence, in the same patient, of characteristic lesions of the 2 entities. This phenomenon may occur synchronously, with clinical features that make differentiation difficult both clinically and histologically, or it may appear at different moments during the patient's disease progression, either spontaneously or triggered by the introduction of biological therapies.1
The latter may be controversial, as some authors do not include it within overlap forms, and it can be found in the literature under other terms such as immune imbalance or the “flip-flop” phenomenon.2 These are patients who, after starting a biological treatment for atopic dermatitis or psoriasis, develop psoriasiform or eczematous lesions respectively. This situation is increasingly relevant due to recent advances in the development of these therapies in modern dermatological practice.3–5
Classically, psoriasis and atopic dermatitis have opposite immunopathogenic mechanisms: psoriasis is characterized by predominant Th1/Th17 inflammation, whereas atopic dermatitis is characterized by Th2 inflammation.6 The coexistence of psoriasis and atopic dermatitis in the same patient has been reported as rare, likely due to the involvement of these opposing inflammatory pathways.1,7,8 However, an increasing number of publications describe patients who present, either concomitantly or at different points in time, clinical features of both diseases.9–12
Although the etiopathogenesis of psoriasiform and eczematous reactions following the initiation of biological therapies is not fully understood, the leading hypothesis relates them to the known cross-inhibitory communication between type 2 (Th2) and type 3 (Th17) immunity.5 Thus, when one arm is inhibited, the other may be inadvertently strengthened. Therefore, in vitro studies in dendritic cells have shown that IL-4 inhibits IL-23 production by dendritic cells.13 A later study using cultured entheseal fibroblasts found that both IL-4 and IL-13 suppressed IL-23 production.14 Additional in vitro studies in macrophages demonstrated that IL-4—and to a lesser extent IL-13—inhibit IL-17–induced macrophage activation.15 These findings suggest that inhibition of IL-4 and/or IL-13 could paradoxically promote IL-23 production and, in doing so, promote Th17 polarization and the onset of psoriasis. Conversely, inhibition of Th17 would block the Th1 pathway and favor an increase in Th2 signaling—involved in the development of eczema—and thus promote typical Th2-mediated reactions, more frequently induced by drugs targeting this interleukin.16,17
Regarding the therapeutic management of these overlapped forms, it is worth noting that we currently have different approved treatments for both atopic dermatitis and psoriasis, some of which are indicated for both diseases. Others, although not formally indicated for one of them, have been traditionally used in both and may be useful for treating combined, difficult-to-manage forms1 (Table 1).
Treatment options for psoriasis, atopic dermatitis, and psoriasis–atopy overlap.
| Treatment | Psoriasis | Atopic dermatitis | Psoriasis–atopy overlap |
|---|---|---|---|
| Topicals | • Corticosteroids• Vitamin D analogs• Retinoids• Calcineurin inhibitorsa | • Corticosteroids• Calcineurin inhibitors | • Corticosteroids• Calcineurin inhibitors |
| Conventional systemic therapies | • Methotrexate• Acitretin• Cyclosporine | • Cyclosporine• Methotrexatea• Azathioprinea | • Cyclosporine• Methotrexatea |
| Phototherapy | • NB-UVB | • NB-UVB | • NB-UVB |
| Biologics | • IL-12/23 inhibitors• IL-17 inhibitors• IL-23 inhibitors• TNF-α inhibitors | • IL-4/13 inhibitors• IL-13 inhibitors | • Combination biologics?• IL-12/23inhibitors?• IL-17 inhibitors? |
| Small-molecule inhibitors | • Phosphodiesterase-4 inhibitor: Apremilast• JAK inhibitors: deucravacitinib• Baricitinib and upadacitinib for psoriatic arthritis | • JAK inhibitors: baricitinib, upadacitinib, abrocitinib | • JAK inhibitors |
Among topical drugs, corticosteroids are approved for both diseases and may be a good alternative for mild forms or in combination with systemic treatments. Calcineurin inhibitors are approved for atopic dermatitis but not for psoriasis; however, they are commonly used because of their long-term safety, especially in locations where topical corticosteroids should be used cautiously in the medium-to-long term, such as the face and skin folds. Vitamin D analogs are indicated for psoriasis but not for atopic dermatitis, and they are not recommended in the latter due to the potential for irritation.
For conventional systemic therapies, several are approved for psoriasis, including cyclosporine, methotrexate, and acitretin. In atopic dermatitis, although multiple systemic agents have been historically used due to the previous scarcity of approved therapies for moderate-to-severe disease, the only conventional systemic drug with an approved indication is cyclosporine. Other commonly used agents include methotrexate, azathioprine, and, in certain cases, mycophenolate mofetil.
Furthermore, phototherapy is indicated for both diseases, with narrow-band UVB being preferred for combined forms.18,19
Regarding biological agents, multiple treatments are approved for psoriasis, such as TNF-α inhibitors, IL-17 inhibitors, IL-12/23 inhibitors, IL-23 inhibitors, among others in the pipeline. For atopic dermatitis, currently approved biologics include IL-4/13 inhibitors (dupilumab) and IL-13 inhibitors (tralokinumab and lebrikizumab). In the reviewed literature, various biological treatment options have been proposed for combined forms, mostly based on isolated cases or small series. A recently published review on psoriasis–atopic dermatitis overlap proposes IL-12/23 inhibitors as treatment.1 However, that same review notes the limited efficacy demonstrated in patients with atopic dermatitis. In a series of 23 patients with atopic dermatitis treated with an IL-12/23 inhibitor (ustekinumab), 8 achieved complete remission, 8 showed no response, and 7 achieved partial remission.20 A phase II clinical trial conducted in Japan to evaluate the efficacy of this same agent in atopic dermatitis found no differences between ustekinumab and placebo at week 24.21
Moreover, the involvement of IL-17 in combined psoriasis–atopy forms has prompted investigation into IL-17 inhibitors as potential treatments.1,6,12 In a phase II clinical trial with secukinumab (an IL-17 inhibitor), no differences were found vs placebo at week 16.22 Interestingly, as intrinsic forms are more strongly associated with elevated IL-17, a subgroup analysis was performed in these patients, again showing no statistically significant differences compared with placebo. No benefit was found either in atopic dermatitis subtypes more common in Asian populations, where IL-17 elevation is also noted.
There is at least 1 isolated case reporting response to another IL-17 inhibitor—brodalumab—in a patient with both dermatological diseases who had been refractory to topical corticosteroids, phototherapy, and methotrexate.23
In another recent review of 30 patients with concomitant atopic dermatitis and psoriasis, the authors describe the treatments used for these patients. They report that 73% failed topical treatments and received systemic therapies during their disease course.24 The most frequently used treatments were dupilumab (43%), guselkumab (27%), adalimumab (27%), cyclosporine (27%), apremilast (20%), secukinumab (20%), ustekinumab (20%), and ixekizumab (20%). Biological therapy was required in 57%, and sequential use of >1 biologic was necessary in 30%. In 22%, at least 2 biologics were required concomitantly to achieve significant clinical improvement. The most frequent combination in that series was guselkumab plus dupilumab, with prolonged combined use reported as safe.
Regarding small-molecule inhibitors, apremilast is approved for psoriasis and, more recently, a TYK2 inhibitor (deucravacitinib). The role of apremilast in the treatment of atopic dermatitis has been investigated.25,26 Although some benefit has been observed in certain patients,25 a phase II clinical trial did not demonstrate superiority over placebo, and therefore its development for this indication has not continued.26 There is an isolated published case in which apremilast was effective in treating psoriasis–dermatitis overlap associated with malignancy.27 Deucravacitinib has not been developed for atopic dermatitis; however, a recent series described seven patients with combined psoriasis/atopy who responded effectively.28 Focusing on small-molecule inhibitors approved specifically for atopic dermatitis, several JAK inhibitors are available (upadacitinib, baricitinib, and abrocitinib). Notably, the first 2 are approved for psoriatic arthritis as well, though not for psoriasis per se. JAK inhibitors act by blocking the signaling of various interleukins through their intracellular receptor-associated pathways.29 Many of these interleukins are involved in atopic dermatitis but also in psoriasis. Thus, an increasing number of publications in the reviewed literature discuss the potential role of JAK inhibitors in overlap forms. Case series and isolated reports describe the use of various JAK inhibitors, including a case series focused specifically on upadacitinib.30–34
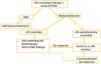
Considering the above, we propose different treatment algorithms for psoriasis–atopic dermatitis overlap. While one could distinguish between true overlap forms (synchronous or alternating) and biologic-induced psoriasis, from a practical standpoint it may be more useful to differentiate between clinical presentations where psoriasis and atopic dermatitis debut synchronously (e.g., lesions compatible with both dermatoses at the same time); and patients with underlying atopic dermatitis on “advanced therapy” (defined as biological drugs or recently approved JAK inhibitors for atopic dermatitis) who develop psoriasiform lesions.
In the first scenario—synchronous onset of both diseases (Table 2)—for mild forms, the most logical initial option is topical corticosteroids and/or calcineurin inhibitors, depending on lesion severity and location. In moderate forms, the treatment of choice may differ depending on whether the presentation reflects an acute exacerbation, in which case cyclosporine may be considered (provided no contraindications exist), or a more chronic–persistent form, in which methotrexate (even though off-label for atopic dermatitis) or phototherapy may be options.
In severe forms or those that do not respond to prior treatments, JAK inhibitors would likely be the agents of choice. Although there is insufficient evidence to select one JAK inhibitor over another, considering their approval for psoriatic arthritis and the benefit shown for skin lesions in such patients, upadacitinib or baricitinib would probably be preferred, with upadacitinib being the most frequently reported in the literature for these cases.
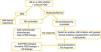
In the second scenario—patients with atopic dermatitis on advanced therapy (biologic or JAK inhibitor) who develop psoriasiform lesions—the treatment algorithm depends on psoriasis severity (Figs. 1 and 2). For mild psoriatic lesions, adding topical corticosteroids is sufficient. For moderate–severe psoriasis where atopic dermatitis is well-controlled, phototherapy or methotrexate may be considered, or, particularly in more severe cases or when previous therapies are contraindicated or ineffective, adding a psoriasis-specific biologic may be required. A different strategy applies when the patient's atopic dermatitis is partially or poorly controlled. If the patient is on a biologic, switching therapeutic targets should be considered. Based on previous discussion, switching to a JAK inhibitor would be appropriate, especially if psoriasis is biologic-induced. If, instead, the patient is already on a JAK inhibitor and atopic dermatitis remains partially or poorly controlled, switching to another JAK inhibitor could be considered (based primarily on case-series evidence suggesting a hierarchy in clinical practice: upadacitinib>baricitinib>abrocitinib,31–33 though current evidence is insufficient to formally support this ranking and decisions should be individualized). If there is still no response after switching, adding a psoriasis biologic to the JAK inhibitor (in cases of partial control) or using biologics for both dermatoses may be considered.
In conclusion, although psoriasis and atopic dermatitis appear to be uncommon in the same patient, increasing reports describe patients with clinical presentations that are difficult to differentiate, or who alternate between both diseases—likely influenced in part by biologic therapies shifting dominant inflammatory responses. Understanding the effectiveness of different therapeutic alternatives for these diseases is crucial to selecting the best treatment option, especially given that many of these patients are complex and often refractory to multiple prior therapies.