Leishmaniasis is a disease caused by parasites of the Leishmania genus.1 Clinically, it can present as cutaneous leishmaniasis, mucocutaneous leishmaniasis, or visceral leishmaniasis.2 The disease is endemic in a number of Spain's autonomous communities, namely, Madrid, Catalonia, Aragon, Castille-la Mancha, Andalusia, Valencia, Extremadura, Murcia, and the Balearic Islands. In some communities it is a notifiable disease, although not all cases are reported.1,3 Clinical manifestations depend on the species of Leishmania, the inoculation site, and the host's immune status.4 Immunosuppressed patients have a high risk of severe disease.5,6
Between 1% and 10% of all cases of cutaneous leishmaniasis spread to the mucous membranes, and almost 90% of these are caused by Leishmania braziliensis, which is rare in our geographic area.
In a retrospective search of the database at our hospital, we identified 18 cases of cutaneous/mucocutaneous leishmaniasis (Table 1). The patients (9 males and 9 females) were aged between 9 and 84 years (mean age, 46 years) and the mean time to diagnosis was 10 months. Six of the 18 patients were immunocompromised for varying reasons (diabetes mellitus, congestive heart failure, liver transplantation, Crohn disease, psoriatic arthritis). Four of them had mucocutaneous leishmaniasis and none of them had been abroad recently.
Clinical and Epidemiological Characteristics of Patients With Cutaneous and Mucocutaneous Leishmaniasis.
| Age (y) and Sex | Lesion Type | No. | Location | Time to Diagnosis (mo) | Origin | Immune Status | Treatment | Outcome at 1 Year |
|---|---|---|---|---|---|---|---|---|
| 20 M | Nodular | 1 | Upper arm | 2 | Autochthonous | Immunocompetent | Excision | Uncertain |
| 62 F | Ulcer | 1 | Lip | 36 | Imported | Immunodepressed (liver transplantation) | Excision and antimoniate | Adequate |
| 12 F | Nodular | 1 | Cheek | 4 | Autochthonous | Immunocompetent | Excision | Uncertain |
| 46 F | Nodular | 1 | Upper arm | 2 | Autochthonous | Immunocompetent | Excision | Adequate |
| 21 M | Psoriasiform | 2 | Upper arm | 8 | Imported | Immunocompetent | None | Adequate |
| 56 M | Psoriasiform/eczematous/papular | 3 | Ear | 6 | Imported | Immunodepressed (Crohn disease under treatment with adalimumab) | Antimoniate | Adequate |
| 45 M | Macular | 1 | Face | 34 | Imported | Immunocompetent | Excision | Adequate |
| 59 F | Ulcer | 3 | Palate | 4 | Autochthonous | Immunodepressed (SLE) | Amphotericin B | Adequate |
| 44 F | Papular | 1 | Cheek | 5 | Autochthonous | Immunocompetent | Excision | Adequate |
| 49 M | Nodular | 3 | Arms and legs | Uncertain | Autochthonous | Immunocompetent | Excision | Adequate |
| 45 M | Large plaque | 1 | Hand | Uncertain | Autochthonous | Immunocompetent | Antimoniate | Adequate |
| 82 F | Macular | 1 | Cheek | 6 | Imported | Immunodepressed (HIV) | Excision | Adequate |
| 42 M | Nodular | 1 | Upper arm | 12 | Autochthonous | Immunocompetent | Imiquimod | Adequate |
| 84 F | Papular | 1 | Eyelid | 12 | Autochthonous | Immunodepressed (DM, CHF) | Excision+cryotherapy | Adequate |
| 9 M | Large plaque | 1 | Leg | 9 | Autochthonous | Immunocompetent | Excision+imiquimod® | Adequate |
| 34 F | Psoriasiform | 3 | Upper arm | 3 | Autochthonous | Immunocompetent | Cryotherapy | Residual lesion |
| 60 M | Papular-nodular | 3 | Sublingual | 3 | Autochthonous | Immunodepressed (psoriatic arthritis under treatment with adalimumab) | Amphotericin B | Adequate |
Abbreviations: CHF, congestive heart failure; F, female; HIV, human immunodeficiency virus; M, male; SLE, systemic lupus erythematosus.
The first patient was a 59-year-old woman with systemic lupus erythematosus under treatment with methotrexate 15mg/wk, hydroxychloroquine 400mg/d, trimethoprim-sulfamethoxazole 160/800mg 3 days a week, acenocoumarol, prednisone, and subcutaneous adalimumab 40mg every 2 weeks. In the week preceding her visit, she had experienced deterioration in her general health and polyarthritis of the carpal bones and knees. Physical examination revealed thickening of the dorsum of the tongue, multiple ulcers on the palate, and erythematous-violaceous papular lesions on her fingers and palms. She was admitted to hospital. A gastric biopsy performed several weeks earlier showed inclusion bodies consistent with Leishmania parasites. Biopsy of the oral mucosa also showed microorganisms consistent with Leishmania. Empirical treatment was started with amphotericin 1mg/kg/d on days 1-5, 10, 17, 24, 31, and 38. Adalimumab therapy was interrupted and the daily dose of prednisone was increased to 20mg. The patient progressed favorably.
Case 2The second patient was a 62-year-old man who had undergone liver transplantation and had a history of chronic kidney failure, gout, and chronic obstructive pulmonary disease. He was being treated with mycophenolate, tiotropium, and allopurinol, and presented with an ulcerated lesion measuring 2cm on the upper lip. The lesion was excised and biopsied, and the histopathology report described a granulomatous lesion with intracellular microorganisms consistent with Leishmania. The patient was prescribed intramuscular meglumine antimonate 20mg/kg/d for 20 days. Response was favorable and he experienced no recurrences.
Case 3The third patient was a 90-year-old woman with high blood pressure, type 2 diabetes mellitus, dyslipidemia, heart failure, obesity, and Parkinson disease. She presented with a hard papular lesion of 1 year's duration accompanied by slight swelling on the lower right eyelid. It was decided to excise the lesion, which had a raised surface area of 1.5×1.5cm and an excavated base involving the conjunctival mucosa. The histopathologic study confirmed the diagnosis of mucocutaneous leishmaniasis. No additional studies or follow-up were performed as the finding was incidental.
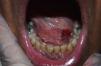
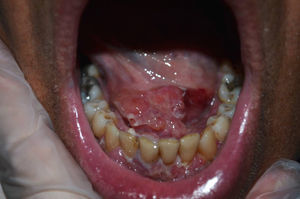
Case 4The fourth case involved a 60-year-old man with psoriatic arthritis under treatment with subcutaneous adalimumab 40mg every 2 weeks and methotrexate 12.5mg every week. He presented with papulonodular lesions of approximately 3 months’ duration in the sublingual region accompanied by gingival enlargement and erosions (Fig. 1). Biopsy of the mucosa showed intracellular microorganisms consistent with Leishmania. Treatment with adalimumab was suspended and replaced with liposomal amphotericin B at a dosage of 3mg/kg/d on days 1 to 5 and days 14 and 21. The treatment was well tolerated and the mucocutaneous lesions resolved.
DiscussionLeishmaniasis is endemic in several autonomous communities in Spain,7 and a rise in incidence has been described in immunodepressed patients.8 Mucosal involvement is very uncommon in the old world,9 and most of the cases reported to date have been in immunocompromised individuals.8 Mucocutaneous involvement is due to L braziliensis in approximately 90% of cases, and this species is found almost exclusively in South America. Nevertheless, there has been a gradual increase in the number of cases involving mucosal lesions caused by Leishmania infantum or Leishmania major.1
In our retrospective review of cases at our hospital, we found that the 4 patients with mucocutaneous leishmaniasis were all immunodepressed, indicating that leishmaniasis must be ruled out in patients with lesions of an unknown origin affecting different mucous membranes. The 4 patients were also all Spanish, which is a noteworthy finding considering that mucocutaneous leishmaniasis is very uncommon in Spain and other parts of Europe.9 Although we were unable to identify the species responsible for the 4 cases described, L braziliensis is a highly unlikely candidate as it is very uncommon in our area.
There is some controversy surrounding the treatment of mucocutaneous leishmaniasis. Although favorable outcomes have been reported following excision, some authors recommend systemic treatment due to the risk of visceral involvement.10,11 Current treatment options are pentavalent antimonials, pentamidine, amphotericin B, azoles, and miltefosine. All immunosuppressive treatments should be suspended until the leishmaniasis has been successfully treated.10 There is also a lack of consensus on the reintroduction of immunosuppressants, (and TNF-α blockers in particular) following treatment due to the risk of recurrence.10 In most of the cases described in the literature, TNF-α blockers were reintroduced following completion of leishmaniasis treatment.
In conclusion, leishmaniasis must be considered in the differential diagnosis of mucosal lesions in immunocompromised patients in leishmania-endemic areas.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Naderizadeh SH, Sierra CV, Gallego LM, Robles BJF, Roustán-Gullón LG. Leishmaniasis mucocutánea en pacientes inmunocomprometidos: reporte de 4 casos autóctonos. Actas Dermosifiliogr. 2018;109:281–284.