Moderate-to-severe atopic dermatitis (AD) is associated with a reduction in the quality of life (QoL) of patients1 and their cohabitants.2,3
The aim of this study was to evaluate the QoL and levels of anxiety and depression in adult patients affected by moderate-to-severe AD and in their cohabitants, as well as the impact of initiating treatment with dupilumab.
We conducted a prospective study including patients who started on dupilumab treatment from February 2020 to April 2021 at Hospital Universitario Virgen de la Victoria (Málaga, Spain). For each patient, one adult cohabitant chosen by the patient during the baseline visit was also included. Cohabitants with other dermatological conditions were excluded.
Only patients older than 18 years with an Eczema Area and Severity Index (EASI)≥21 and a lack of response, intolerance, or contraindication to cyclosporine treatment were included. Patients who started dupilumab within a clinical trial and did not have an eligible adult cohabitant were excluded.
Dupilumab was administered according to the approved product label. Patients on systemic therapy for AD reduced their dosage upon initiation of dupilumab and discontinued it before 12 weeks, while continuing the use of emollients, corticosteroids, or topical calcineurin inhibitors.
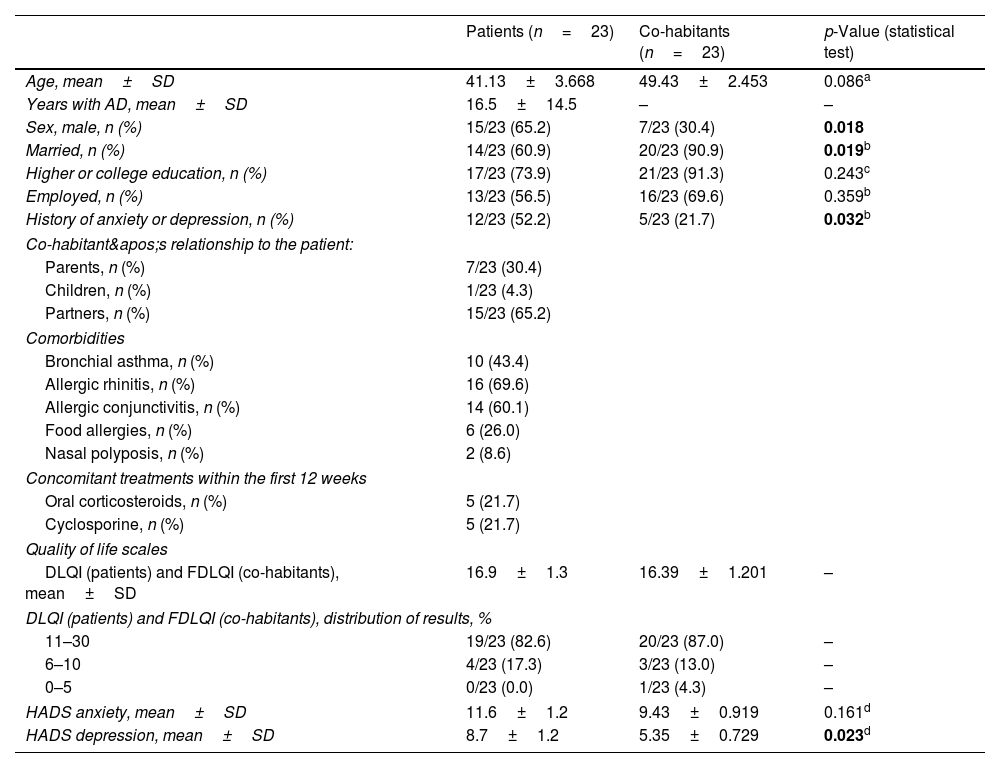
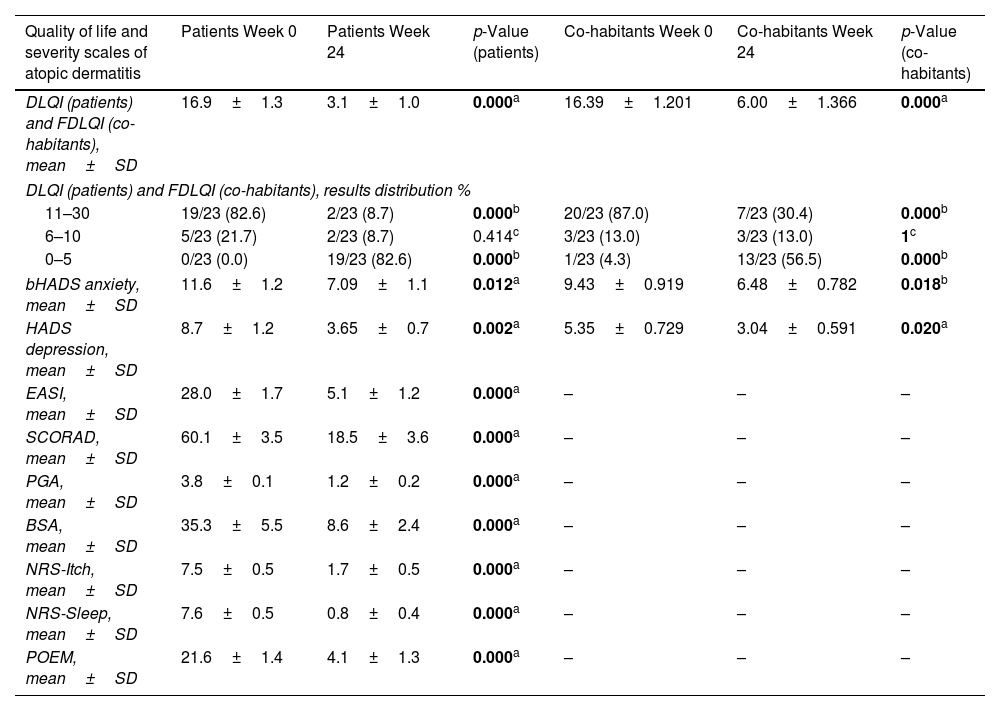
A total of 23 patients and their respective cohabitants were included. Both groups were assessed by a dermatologist at the start of dupilumab treatment and again 24 weeks later. Baseline characteristics, severity scales, and QoL measures are shown in Tables 1 and 2.
Demographic, clinical characteristics, and quality of life scales of the 2 study samples.
| Patients (n=23) | Co-habitants (n=23) | p-Value (statistical test) | |
|---|---|---|---|
| Age, mean±SD | 41.13±3.668 | 49.43±2.453 | 0.086a |
| Years with AD, mean±SD | 16.5±14.5 | – | – |
| Sex, male, n (%) | 15/23 (65.2) | 7/23 (30.4) | 0.018 |
| Married, n (%) | 14/23 (60.9) | 20/23 (90.9) | 0.019b |
| Higher or college education, n (%) | 17/23 (73.9) | 21/23 (91.3) | 0.243c |
| Employed, n (%) | 13/23 (56.5) | 16/23 (69.6) | 0.359b |
| History of anxiety or depression, n (%) | 12/23 (52.2) | 5/23 (21.7) | 0.032b |
| Co-habitant's relationship to the patient: | |||
| Parents, n (%) | 7/23 (30.4) | ||
| Children, n (%) | 1/23 (4.3) | ||
| Partners, n (%) | 15/23 (65.2) | ||
| Comorbidities | |||
| Bronchial asthma, n (%) | 10 (43.4) | ||
| Allergic rhinitis, n (%) | 16 (69.6) | ||
| Allergic conjunctivitis, n (%) | 14 (60.1) | ||
| Food allergies, n (%) | 6 (26.0) | ||
| Nasal polyposis, n (%) | 2 (8.6) | ||
| Concomitant treatments within the first 12 weeks | |||
| Oral corticosteroids, n (%) | 5 (21.7) | ||
| Cyclosporine, n (%) | 5 (21.7) | ||
| Quality of life scales | |||
| DLQI (patients) and FDLQI (co-habitants), mean±SD | 16.9±1.3 | 16.39±1.201 | – |
| DLQI (patients) and FDLQI (co-habitants), distribution of results, % | |||
| 11–30 | 19/23 (82.6) | 20/23 (87.0) | – |
| 6–10 | 4/23 (17.3) | 3/23 (13.0) | – |
| 0–5 | 0/23 (0.0) | 1/23 (4.3) | – |
| HADS anxiety, mean±SD | 11.6±1.2 | 9.43±0.919 | 0.161d |
| HADS depression, mean±SD | 8.7±1.2 | 5.35±0.729 | 0.023d |
AD: atopic dermatitis; DLQI: Dermatology Life Quality Index; FDLQI: Family Dermatology Life Quality Index; HADS: Hospital Anxiety and Depression Score; SD: standard deviation.
Bold indicates statistically significant values (p<0.05).
Quality of life and atopic dermatitis severity scales before the start of treatment and 24 weeks into dupilumab.
| Quality of life and severity scales of atopic dermatitis | Patients Week 0 | Patients Week 24 | p-Value (patients) | Co-habitants Week 0 | Co-habitants Week 24 | p-Value (co-habitants) |
|---|---|---|---|---|---|---|
| DLQI (patients) and FDLQI (co-habitants), mean±SD | 16.9±1.3 | 3.1±1.0 | 0.000a | 16.39±1.201 | 6.00±1.366 | 0.000a |
| DLQI (patients) and FDLQI (co-habitants), results distribution % | ||||||
| 11–30 | 19/23 (82.6) | 2/23 (8.7) | 0.000b | 20/23 (87.0) | 7/23 (30.4) | 0.000b |
| 6–10 | 5/23 (21.7) | 2/23 (8.7) | 0.414c | 3/23 (13.0) | 3/23 (13.0) | 1c |
| 0–5 | 0/23 (0.0) | 19/23 (82.6) | 0.000b | 1/23 (4.3) | 13/23 (56.5) | 0.000b |
| bHADS anxiety, mean±SD | 11.6±1.2 | 7.09±1.1 | 0.012a | 9.43±0.919 | 6.48±0.782 | 0.018b |
| HADS depression, mean±SD | 8.7±1.2 | 3.65±0.7 | 0.002a | 5.35±0.729 | 3.04±0.591 | 0.020a |
| EASI, mean±SD | 28.0±1.7 | 5.1±1.2 | 0.000a | – | – | – |
| SCORAD, mean±SD | 60.1±3.5 | 18.5±3.6 | 0.000a | – | – | – |
| PGA, mean±SD | 3.8±0.1 | 1.2±0.2 | 0.000a | – | – | – |
| BSA, mean±SD | 35.3±5.5 | 8.6±2.4 | 0.000a | – | – | – |
| NRS-Itch, mean±SD | 7.5±0.5 | 1.7±0.5 | 0.000a | – | – | – |
| NRS-Sleep, mean±SD | 7.6±0.5 | 0.8±0.4 | 0.000a | – | – | – |
| POEM, mean±SD | 21.6±1.4 | 4.1±1.3 | 0.000a | – | – | – |
AD: atopic dermatitis; BSA: body surface area; DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; FDLQI: Family Dermatology Quality of Life Index; HADS: Hospital Anxiety and Depression Scale; NRS: Numerical Rating Scale; PGA: Physician Global Assessment; POEM: Patient-Oriented Eczema Measure; SCORAD: Scoring Atopic Dermatitis; SD: standard deviation.
Bold indicates statistically significant values (p<0.05).
Statistical analysis was performed using IBM SPSS 22.0, with means and standard deviations for quantitative variables, and frequencies and percentages for categorical variables. Comparative analysis was conducted using the Mann–Whitney test, Pearson's Chi-square test, Fisher's exact test, or Levene's test as appropriate. A 95% confidence interval was used.
Recent articles indicate that chronic dermatological conditions such as AD, psoriasis, or acne can impact the QoL of patients and their cohabitants.4,5
In the case of AD, special attention has been paid to the impact on the QoL of parents of affected children. For example, the study by Marciniak et al. investigated a sample of children aged 2–24 months with AD and their parents’ QoL.2 Parental QoL was affected, especially in mothers, with a mean Family Dermatology Life Quality Index (FDLQI) score of 17.1±5.3 vs 14.7±5.8 points; p<0.001.
Similar studies have been conducted in adolescents, such as the one by Ezzedine et al., which evaluated QoL in 399 adolescents aged 12–17 with AD and their parents.3 A reduction in parental QoL was observed, with a moderate effect in the younger group (12–14 years), and a greater impact in the parents of adolescents aged 15–17, showing a worsening of parental QoL with increasing child age.
However, as far as we know, no other studies have analyzed how AD affects the QoL of adult patients’ cohabitants. In our sample, a moderate-to-severe impairment in QoL was observed in 100% of patients and 95.6% of cohabitants, as measured by the DLQI (16.9±1.3) and FDLQI (16.39±1.201), respectively.
These results suggest that AD affects not only the patients’ QoL and mental health but also that of their cohabitants and caregivers, who might benefit from a multidisciplinary approach including psychological support.
Study limitations include the small sample size, lack of a control group, and a follow-up period limited to 24 weeks. Strengths include its prospective design and the novel focus on the impact of AD treatment on the QoL of cohabitants.
Conflicts of interestNone declared.