Atopic dermatitis (AD) is a chronic skin disease that may be triggered by psychological conditions and several allergens. Patients with AD may be experienced disease exacerbation due to the COVID-19 pandemic lifestyle including home-quarantine and increased stress. We obtained the electronic data of 100 AD patients admitted to our hospital from 2016 to 2019 and called them with specific phone line.
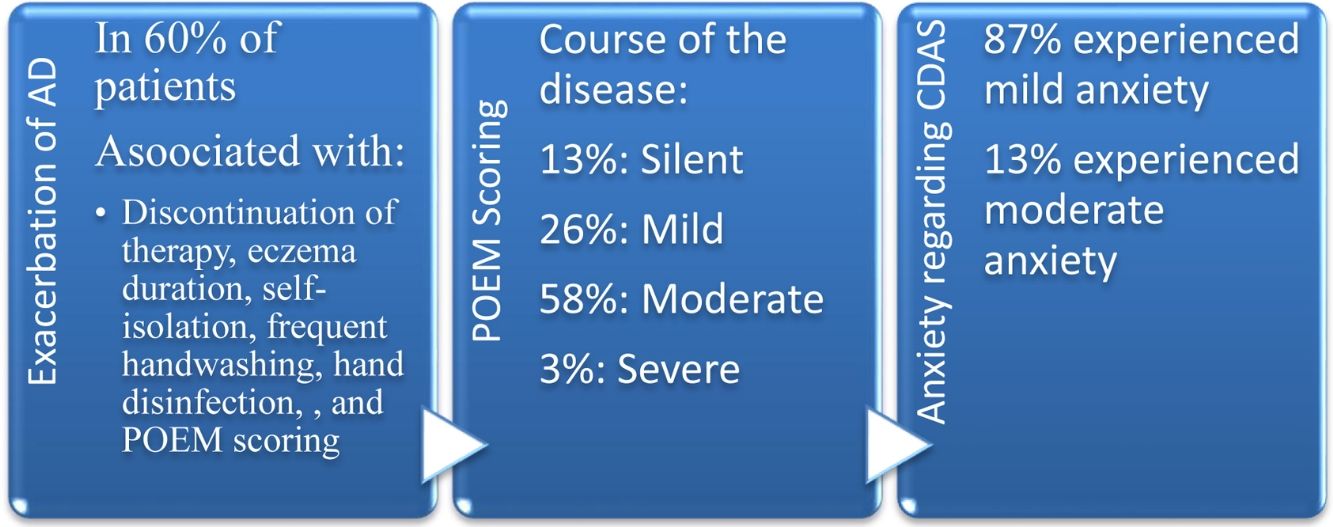
Out of 100 patients, 43 were male, and 57 were female (mean age±SD: 45.85±16.90). Sixty patients (37 females and 23males; mean age: 42.22±14.71) confronted disease flare-up during the COVID-19 era. Exacerbation of AD was correlated with treatment dose alteration, a lengthy history of atopic dermatitis, eczema duration, self-isolation, frequent handwashing, hand disinfection, and POEM scoring (P<0.05). Regarding the POEM scoring, 61 patients with moderate to severe AD experienced higher anxiety than 39 patients with silent to mild AD (P=0.013).
In this study, most patients experienced disease exacerbation and perceived mild anxiety in this pandemic.
La dermatitis atópica (DA) es una enfermedad cutánea crónica que puede desencadenarse debido a situaciones psicológicas y ciertos alérgenos. Los pacientes con DA pueden haber experimentado una exacerbación de la enfermedad debido al estilo de vida durante la pandemia de la COVID-19, incluyendo el confinamiento domiciliario y el incremento del estrés. Obtuvimos los datos electrónicos de 100 pacientes con DA ingresados en nuestro hospital de 2016 a 2019, y les llamamos con una línea telefónica específica.
De los 100 pacientes, 43 eran varones y 57 mujeres (edad media ± DE: 45,85 ± 16,90), de los cuales 60 (37 mujeres y 23 varones, con edad media de 42,22 ± 14,71) experimentaron el brote de la enfermedad durante la etapa de la COVID-19. La exacerbación de la DA guardó relación con la alteración de la dosis de tratamiento, un largo historial de dermatitis atópica, la duración del eccema, el autoaislamiento, la frecuencia del lavado de manos, la desinfección de las manos, y la puntuación POEM (p < 0,05). En lo referente a dicha puntuación, los 61 pacientes con DA de moderada a grave experimentaron mayor ansiedad que los 39 pacientes con DA de silente a leve (p = 0,013).
En este estudio muchos pacientes experimentaron exacerbación de la enfermedad y percibieron ansiedad leve durante la pandemia.
In December 2019, Wuhan became the center of an outbreak of pneumonia of unknown cause, which was later nominated as coronavirus disease-2019 (COVID-19) by the World Health Organization (WHO).1 Risk factors associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection severity include Old age, male gender, and chronic comorbidities including diabetes mellitus and hyprtension.2
Atopic dermatitis (AD) is a chronic relapsing cutaneous disease that may be exacerbated by several triggers, including stress, some allergens, and infections. Arndt et al. proposed psycho-neuro-immunologic factors and stress as essential elements in AD evolution.3 Stress significantly influences the immune system activity and the course of AD; for instance, in another study, AD flare-up was associated with subjective distress.4
AD patients like those with other chronic diseases may encounter various challenges in the COVID-19 pandemic.5 Some patients require systemic immunosuppressive treatment to control the course of the disease. Furthermore, decreased immune system function and possible exposure to SARS-CoV-2 infection may increase the likelihood of COVID-19, particularly in those with chronic illness and simultaneous use of systemic medication.6,7 However, some studies recommended to continue immunosuppressive medication in absence of COVID-19-related symptoms.8 The frequent handwashing and recommended protective measures against COVID-19 and stress during quarantine may lead to the exacerbation or recurrence of symptoms in patients with AD.9 Therefore, we aimed to accomplish a teledermatology study on AD patients in the COVID-19 era.
Material and methodsWe obtained the electronic data of 150 AD patients who were admitted to our hospital from July 2016 to September 2019 and contacted them by phone-call.
All subjects were asked about their adherence to health principles, developing COVID-19 symptoms, AD exacerbation symptoms, AD severity according to POEM questionnaire,10 and anxiety based on the Corona Disease Anxiety Scale (CDAS)11 during the COVID-19 pandemic. The POEM includes seven questions regarding the number of days each symptom appeared (itch, sleep disturbance, weeping/oozing, cracking, flaking, and dryness/roughness). Each question is scored from 0 to 4. POEM scores are categorized as follows: 0–2=clear/almost clear, 3–7=mild, 8–16=moderate, 17–24=severe, 25–28=very severe. The Corona Disease Anxiety Scale consists of 18 questions (nine questions related to physical symptoms of anxiety and the other nine questions about psychological symptoms of anxiety). This scale ranges between 0 and 54 (each question scored from 0 to 3) and high scores demonstrated an increased level of anxiety in patients. Patients with confirmed COVID-19 were asked further questions about the severity of the disease as well as the treatment protocol.
The continuous variables and categorical variables results were reported as Mean±SD and frequency plus a percentage, respectively. The Chi-square and Fisher exact tests were applied to assess the correlation between categorical variables. The level of significance for statistical tests was 0.05. The independent t-test was used to compare the means of continuous variables. For statistical analysis, we used The SPSS software version 25.
ResultsOut of 150 patients, 10 cases had died over the past years (for reasons like old age, MI, accidents, and so on.), and 35 cases did not answer the call. Five patients were not satisfied with participating in the study. Finally, 100 patients were enrolled in the study.
Out of a total of 100 patients, 43 were male, and 57 were female (mean age±SD: 45.85±16.90). The three most common sites of involvement with AD included hands, posterior trunk, and neck, respectively. According to the POEM questionnaire, 13%, 26%, 58%, 3%, and 0% of patients had comprehended the silent, mild, moderate, severe, and very severe course of the disease, respectively. Oral prednisolone, cyclosporine, and azathioprine were the most commonly used oral immunosuppressive medications. A number of patients had discontinued their medication during the pandemic due to concerns about acquisition of COVID-19 (7%) and the closure of clinics (13%). Ninety patients kept home quarantine studiously; 88 (88%) and 61 (61%) patients used face masks and applied gloves in public places, respectively.
Sixty patients (37 females and 23 males; mean age: 42.22±14.71) developed pruritus and experienced disease exacerbation during this pandemic (Table 1). Patients with worsening of the disease were statistically younger than those with no disease exacerbation (42.22±14.71 vs. 51.30±18.62, respectively; p value=0.012)
The demographic data of all AD patients.
| Exacerbated AD patients(n=60) | Non-exacerbated AD patients(n=40) | P | |
|---|---|---|---|
| Sex | |||
| Male | 23 (38.3%) | 20 (50%) | 0.248 |
| Female | 37 (61.7%) | 20 (50%) | |
| Age (years) Mean±SD | 42.217±14.713 | 51.300±18.622 | 0.012 |
| Marital status | |||
| Single | 23 (38.3%) | 7 (17.5%) | 0.026 |
| Married | 37 (61.7%) | 33 (82.5%) | |
| Educational level | |||
| Diploma or lower | 9 (15%) | 15 (37.5%) | 0.007 |
| B.Sc./B.A. | 32 (53.3%) | 21 (52.5%) | |
| MSc, MD, Ph.D. or higher | 19 (31.7%) | 4 (10%) | |
| History of alcohol consumption | |||
| Yes | 3 (5%) | 1 (2.5%) | 0.532 |
| No | 57 (95%) | 39 (97.5%) | |
| History of smoking cigarette | |||
| Yes | 3 (5%) | 4 (10%) | 0.337 |
| No | 57 (95%) | 36 (90%) | |
| Familial history of Eczema | |||
| Yes | 29 (48.3%) | 7 (17.5%) | 0.002 |
| No | 31 (51.7%) | 33 (82.5%) | |
| Chronic comorbiditiesa | |||
| Yes | 39 (65%) | 21 (52.5%) | 0.211 |
| No | 21 (35%) | 19 (47.5%) | |
| History of food/drug allergy | |||
| Yes | 38 (63.3%) | 25 (62.5%) | 0.933 |
| No | 22 (36.7%) | 15 (37.5%) | |
| Topical treatment | |||
| Topical corticosteroids | 2 (3.3%) | 5 (12.5%) | 0.030 |
| Eucerin/vaseline | 1 (1.7%) | 4 (10%) | |
| More than 1 topicalb including steroids | 50 (83.3%) | 30 (75%) | |
| More than 1 topical without steroids | 7 (11.7%) | 1 (2.5%) | |
| Phototherapy | |||
| Yes | 33 (55%) | 11 (27.5%) | 0.007 |
| No | 27 (45%) | 29 (72.5%) | |
| Systemic treatment | |||
| Immunosuppressive medicationc | 29 (48.3%) | 12 (30%) | 0.001 |
| Antihistamine | 6 (10%) | 17 (42.5%) | |
| No | 25 (41.7%) | 11 (27.5%) | |
| Phototherapy discontinuation | |||
| Yes | 52 (86.7%) | 32 (80%) | 0.373 |
| No | 8 (13.3%) | 8 (20%) | |
| Treatment discontinuation | |||
| Yes | 17 (28.3%) | 4 (10%) | 0.027 |
| No | 43 (71.7%) | 36 (90%) | |
| Treatment dose alteration | |||
| Yes, increased | 11 (18.3%) | 1 (2.5%) | <0.001 |
| Yes, decreased | 22 (36.7%) | 0 (0%) | |
| No | 27 (45%) | 39 (97.5%) | |
| The disease duration (years) Mean±SD | 12.567±8.472 | 7.225±6.224 | <0.001 |
| Self-quarantine | |||
| No | 3 (5%) | 7 (17.5%) | <0.001 |
| Most times | 22 (36.7%) | 32 (80%) | |
| Always | 35 (58.3%) | 1 (2.5%) | |
| Handwashing (more than 10 times/day) | |||
| No | 2 (3.3%) | 5 (12.5%) | <0.001 |
| Most times | 10 (16.7%) | 30 (75%) | |
| Always | 48 (80%) | 5 (12.5%) | |
| Hand disinfection | |||
| Yes | 49 (81.7%) | 17 (42.5%) | <0.001 |
| No | 11 (18.3%) | 23 (57.5%) | |
| Surface disinfection | |||
| Yes | 4 (6.7%) | 10 (25%) | 0.010 |
| No | 56 (93.3%) | 30 (75%) | |
| History of COVID-19 infection | |||
| Yes | 3 (5%) | 0 (0%) | 0.151 |
| No | 57 (95%) | 40 (100%) | |
| POEM scoring | |||
| Silent | 1 (1.7%) | 12 (30%) | <0.001 |
| Mild | 10 (16.7%) | 16 (40%) | |
| Moderate | 46 (76.7%) | 12 (30%) | |
| Severe | 3 (5%) | 0 (0%) | |
| Very severe | 0 (0%) | 0 (0%) | |
| The Corona disease anxiety scale Mean±SD | 6.883±5.696 | 10.775±6.154 | 0.001 |
Abbreviations: atopic dermatitis, AD; Bachelor of Arts, B.A.; Bachelor of Science, B.Sc.; coronavirus disease 2019, COVID-19; Master of Science, MSc; medical doctor, MD; number, n; doctoral degree, Ph.D.
Aggravation of AD was associated with marital status, educational level, familial history of eczema, prolonged history of atopic dermatitis, discontinuation of therapy, treatment dose alteration, eczema duration, self-isolation, frequent handwashing (more than 10 times per day), hand disinfection, surface sanitization, and POEM scoring (P<0.05). Atopic dermatitis flare-up was not correlated with a history of alcohol consumption and smoking cigarette, chronic comorbidities, allergy to drug or food, discontinuation of phototherapy, and COVID-19 acquisition. Based on the CDAS, 87% and 13% of subjects experienced mild and moderate anxiety, respectively. Three out of 100 AD patients afflicted with SARS-CoV-2; the characteristics of the confirmed COVID-19 cases are summarized in Table 2.
Characteristics of AD patients with COVID-19.
| Number patient | 1 | 2 | 3 |
|---|---|---|---|
| Gender | Female | Female | Female |
| Age | 40 | 33 | 29 |
| Education | Diploma | B.Sc. | B.Sc. |
| Job | Housewife | Unemployment | Not mentioned |
| Marital status | Married | Single | Single |
| Smoking cigarette | No | No | No |
| Alcohol consumption | No | No | No |
| Comorbidities | Hypothyroidism | No | No |
| AD exacerbation | Yes | Yes | Yes |
| Immunosuppressive treatment | Azathioprine | No | More than 1 drug with prednisolone |
| Mask usage | No | Yes, clothes | Yes, surgical |
| Glove usage | No | Yes, Nylon | No |
| Home quarantine | Always | Always | Usually |
| Hand washing | Always | Always | Always |
| Hand disinfection | Yes | Yes | Yes |
| Surface sanitization | Yes | Yes | Yes |
| Signs of COVID-19 | Fever, myalgia, malaise | Anosmia, fever, diarrhea | Fever, dry cough, myalgia, malaise |
| Lab data abnormality | No | No | Lymphopenia, increased ESR |
| Treatments for COVID-19 | Hydroxychloroquine | Hydroxychloroquine | Hydroxychloroquine+azithromycin |
| Close contact | Yes, in common places | Yes, in common places | Yes, close contact |
| Travel history | No | No | No |
| POEM | Moderate | Moderate | Mild |
| Corona disease anxiety score | 5 | 4 | 2 |
Abbreviations: atopic dermatitis, AD; Bachelor of Science, B.Sc.; coronavirus disease 2019, COVID-19; erythrocyte sedimentation rate, ESR.
According to the POEM scoring system, 61 patients with moderate to severe AD had higher anxiety than 39 patients with silent to mild AD (P=0.013). Moderate to severe course of the disease was associated with a history of smoking, familial history of eczema, chronic comorbidities, history of food/drug allergy, hand washing and disinfection, diminished moisturizing usage, and taking immunosuppressive medication (P<0.05) (Table 3).
The demographic data of patients with different course of atopic dermatitis.
| Silent to mild AD (n=39) | Moderate to severe AD (n=61) | P | |
|---|---|---|---|
| Sex | |||
| Male | 21 (53.8%) | 22 (36.1%) | 0.080 |
| Female | 18 (46.2%) | 39 (63.9%) | |
| Age (years) Mean±SD | 53.51±17.98 | 40.95±14.28 | <0.001 |
| Marital status | |||
| Single | 8 (20.5%) | 22 (36.1%) | 0.098 |
| Married | 31 (79.5%) | 39 (63.9%) | |
| Educational level | |||
| Diploma or lower | 13 (33.3%) | 11 (18%5) | 0.197 |
| B.Sc./B.A. | 19 (48.7%) | 34 (55.7%) | |
| MSc, MD, Ph.D. or higher | 7 (17.9%) | 16 (26.2%) | |
| History of alcohol consumption | |||
| Yes | 0 (0%) | 4 (6.6%) | 0.103 |
| No | 39 (100%) | 57 (93.4%) | |
| History of smoking | |||
| Yes | 5 (12.8%) | 2 (3.3%) | 0.068 |
| No | 34 (87.2%) | 59 (96.7%) | |
| Familial history of eczema | |||
| Yes | 9 (23.1%) | 27 (44.3%) | 0.031 |
| No | 30 (76.9%) | 34 (55.7%) | |
| AD exacerbation | |||
| Yes | 11 (28.2%) | 49 (80.3%) | <0.001 |
| No | 28 (71.8%) | 12 (19.7%) | |
| Chronic comorbiditiesa | |||
| Yes | 18 (46.2%) | 42 (68.9%) | 0.024 |
| No | 21 (53.8%) | 19 (31.1%) | |
| History of food/drug allergy | |||
| Yes | 19 (48.7%) | 44 (72.1%) | 0.018 |
| No | 20 (51.3%) | 17 (27.9%) | |
| Topical treatment | |||
| Topical steroids | 5 (12.8%) | 2 (3.3%) | 0.050 |
| Eucerin/vaseline | 4 (10.3%) | 1 (1.6%) | |
| More than 1 topicalb including steroids | 28 (71.8%) | 52 (85.2%) | |
| More than 1 topical without steroids | 2 (5.1%) | 6 (9.8%) | |
| Phototherapy | |||
| Yes | 13 (33.3%) | 31 (50.8%) | 0.086 |
| No | 26 (66.7%) | 30 (49.2%) | |
| Systemic treatment | |||
| No immunosuppressive medicationc | 28 (71.8%) | 31 (50.8%) | 0.038 |
| Immunosuppressive medication | 11 (28.2%) | 30 (49.2%) | |
| Phototherapy discontinuation | |||
| Yes | 31 (79.5%) | 53 (86.9%) | 0.325 |
| No | 8 (20.5%) | 8 (13.1%) | |
| Treatment discontinuation | |||
| Yes | 5 (12.8%) | 16 (26.2%) | 0.108 |
| No | 34 (87.2%) | 45 (73.8%) | |
| Treatment dose alteration | |||
| Yes, increased | 4 (10.3%) | 8 (13.1%) | 0.014 |
| Yes, decreased | 3 (7.7%) | 19 (31.1%) | |
| No | 32 (82.1%) | 34 (55.7%) | |
| Moisturizing usage | |||
| Yes | 17 (43.6%) | 52 (85.2%) | <0.001 |
| No | 22 (56.4%) | 9 (14.8%) | |
| The disease duration (years) Mean±SD | 7.87±6.55 | 12.06±8.54 | 0.010 |
| Self-quarantine | |||
| No | 7 (17.9%) | 3 (4.9%) | 0.001 |
| Most times | 26 (66.7%) | 28 (45.9%) | |
| Always | 6 (15.4%) | 30 (49.2%) | |
| Handwashing | |||
| No | 5 (12.8%) | 2 (3.3%) | 0.004 |
| Most times | 21 (53.8%) | 19 (31.1%) | |
| Always | 13 (33.3%) | 40 (65.6%) | |
| Hand disinfection | |||
| Yes | 19 (48.7%) | 47 (77%) | 0.004 |
| No | 20 (51.3%) | 14 (23%) | |
| Surface disinfection | |||
| Yes | 31 (79.5%) | 55 (90.2%) | 0.133 |
| No | 8 (20.5%) | 6 (9.8%) | |
| History of COVID-19 infection | |||
| Yes | 1 (2.6%) | 2 (3.3%) | 0.838 |
| No | 38 (97.4%) | 59 (96.7%) | |
| Total anxiety | |||
| Mild | 38 (97.4%) | 49 (80.3%) | 0.013 |
| Moderate | 1 (2.6%) | 12 (19.7%) | |
| Severe | 0 | 0 | |
| The Corona disease anxiety scale Mean±SD | 9.87±6.76 | 7.52±5.61 | 0.107 |
Abbreviations: atopic dermatitis, AD; Bachelor of Arts, B.A.; Bachelor of Science, B.Sc.; Coronavirus disease 2019, COVID-19; Master of Science, MSc; medical doctor, MD; number, n; doctoral degree, Ph.D.
In this study, forty-one patients received immunosuppressive medication. Patients under immunosuppressant agents discontinued or altered their treatment schedule (P=0.007, P<0.001; respectively); and also consumed vegetables and supplements more than patients without immunosuppressive therapy in the COVID-19 era (85.4% vs. 66.1, P=0.031, 48.8% vs. 25.4%, P=0.016; respectively).
DiscussionIn the COVID-19 pandemic, millions of people are compelled to be home-quarantined, leading to several psychological issues, including anxiety and depression. Psychological stress is one of the most important triggers for AD flare-up and correlates with increased sensation of itching that may aggravate the disease.12,13 Based on the CADS, most patients in our study perceived mild anxiety in this pandemic. However, 60% of AD patients experienced disease exacerbation in the COVID-19 pandemic. A review of the literature demonstrated higher exacerbation and worsening course of the disease in AD patients were associated with some stressors.3,4 Participating in the virtual support group, increasing daily activities including regular exercise, and teaching techniques for decreasing stress are the best recommendations for improving psychological conditions.6 Some modalities for control of stress and anxiety have been proposed, including relaxation therapy, hypnosis, and psychotherapies like cognitive-behavioral therapy.3
Williams et al. reported that irritant agents play an essential role in AD aggravation. Escalated use of soap and hard water can exacerbate disease due to impaired epidermal function and increased exposure to irritants.14 In addition, Shah et al. highlighted the tremendous effects of COVID-19 on chronic dermatological disorders like atopic dermatitis, noting that the use of preventive measures could lead to higher rates of hand and facial dermatitis that might exacerbate the course of atopic dermatitis.6
We found that increased handwashing, hand disinfection, and surface sanitization were associated with AD exacerbation. Furthermore, 88% of AD patients utilized face masks, and 61% used gloves. These findings offered the importance of following health principles and the role of physicians and health workers in increasing social awareness about COVID-19 preventive measures. Some strategies for the prevention of atopic dermatitis flares in the COVID-19 pandemic should be considered, including proper hand-washing procedures like usage of warm water and creamy soap, frequent application of hand cream and moisturizers, utilization of gentle hand sanitizers and cleansers, consumption of cotton gloves beneath other gloves in washing dishes and cleaning surfaces, and employment of fragrance-free detergents.6,15,16 In this survey, patients with moderate to severe AD used moisturizing cream more than patients with silent to mild course of AD. Recent studies emphasized on importance of moisturizers as a preventive measure against dermatitis.
In this pandemic, tele-dermatology platform could decrease the emergency department visits as well as the possibility of COVID-19 acquisition, especially in those under immunosuppressive agents.6 The tele-dermatology visits should be reinforced to provide the best care of these patients; precise evaluations of the course of the disease and assessments of psychological conditions should be performed to prevent the disease flare and possible secondary infections.
The authors recommended minimizing the dose of prednisolone or other prednisolone-sparing immunosuppressants to diminish the possible risk of SARS-CoV-2 infection.6 In our investigation, the discontinuation and alteration of treatment were associated with AD exacerbation. The minimum effective dose of treatment that controls the disease may reduce unnecessary visits for monitoring of drug-related side effects and consequently the risk of exposure to SARS-CoV-2 infection.6
This survey is the first study to evaluate impact of the COVID-19 pandemic on patients with AD to the best of our knowledge. However, the small sample size and lack of control group were the major limitations of our investigation. In addition, regarding absence of subjects with severe anxiety, determination of relation of stress to AD exacerbation was not practicable in this study. This pandemic emerged as a unique situation to assess the effect of COVID-19 on dermatological diseases like AD.
The COVID-19 era highlights the importance of teledermatology in the follow-up of patients with recognized diseases. Therefore, all dermatologists should provide an exclusive line to investigate the patient's course of disease in this pandemic. Besides, the consideration of psychological consultation for anxiety, depression, and so on for patients with chronic diseases such as AD plays a significant role in the management of the burden of disease. Suggesting proper preventive measures and health recommendations in teledermatology visits assist patients to alleviate their psychological condition and possible disease flare-up.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors report no conflict of interest.
None.