Although invasive techniques such as scalp biopsies and non-invasive techniques such as trichoscopy are commonly used for the diagnosis and management of alopecia, the latter does not always provide enough information on the state of the disease. Currently, we have new non-invasive diagnostic methods to complement the clinical and trichoscopic findings of patients with alopecia; these are high-frequency ultrasound (HFU), ultra-high-frequency ultrasound (UHFU), reflectance confocal microscopy (RCM) and optical coherence tomography (OCT), which are capable of identifying structures under the surface of the scalp, while maintaining the ability to monitor hair shafts.
This review describes the main features reported with each technique for the different types of alopecia—scarring and non-scarring—with the aim of identifying those parameters that may contribute to clarifying new pathophysiological findings, while adding information to the trichoscopic examination and improving diagnostic processes and monitoring.
Para el diagnóstico y manejo de las alopecias se utilizan técnicas invasivas, como biopsias de cuero cabelludo, y no invasivas, como tricoscopia, pero esta no siempre proporciona suficiente información sobre el estado de la enfermedad. En la actualidad, disponemos de nuevos métodos diagnósticos no invasivos para complementar los hallazgos clínicos y tricoscópicos de pacientes con alopecia. Estos son la ecografía de alta frecuencia (EAF) y ultraalta frecuencia (EUAF), la microscopia confocal de reflectancia (MCR) y la tomografía de coherencia óptica (TCO), que son capaces de identificar estructuras bajo la superficie del cuero cabelludo, manteniendo la capacidad de monitorizar los tallos pilosos. En esta revisión se describen las características reportadas con cada técnica para los distintos tipos de alopecias (cicatriciales y no cicatriciales), con el objetivo de identificar parámetros que contribuyan a aclarar nuevos hallazgos fisiopatológicos, sumar información al examen tricoscópico y mejorar los procesos de diagnóstico y seguimiento.
The diagnostic approach to the different types of alopecia is mainly based on the clinical history, physical examination, trichoscopy, and histopathological study. The search for new diagnostic methods, especially non-invasive ones, is increasingly relevant, as they provide information for differential diagnosis, contribute to therapeutic decisions, and reduce the need for biopsies.1–4 In recent years, there has been increasing interest in techniques such as high-frequency ultrasound (HFUS), reflectance confocal microscopy (RCM), and optical coherence tomography (OCT), as they have shown utility in their diagnosis and monitoring. This review describes the main evidence compiled regarding the use of these techniques in trichology, in both non-scarring and scarring alopecias.
Regarding methodology, an electronic search was performed to collect publications on the use of HFUS, RCM, and OCT in the evaluation, diagnosis, and monitoring in trichology. The review was conducted until March 2024 in the PubMed electronic database using the search terms “high-frequency ultrasound,” “reflectance confocal microscopy,” “optical coherence tomography,” “alopecia,” and “hair.”
UltrasoundHFUS is a widely used imaging modalitu in dermatology. It is based on the emission of high-frequency ultrasonic pulses (>15MHz) from a transducer onto the skin, which records their return (echo phenomenon) in the processing unit. The minimum frequency recommended for the study of skin structures varies between 15 and 20MHz1 with depths between 6mm and 10mm. Devices emitting waves>50MHz are called ultra-high frequency ultrasound (UHFUS) and can currently reach up to 70MHz.5 The difference between the 2 is the axial spatial resolution, which is higher in UHFUS and reaches 30μm. In addition, through color Doppler mode, the speed of blood flow can be detected and measured.
In the field of trichology—normal hair follicles are described ultrasonographically as oblique hypoechoic bands in the dermis that, in the anagen phase—are located in the deep dermis or even in the upper hypodermis, while in the telogen phase they are restricted to the upper dermis (Fig. 1). In the scalp, hair shafts are reported as predominantly trilaminar hyperechoic structures with an outer cuticle-cortex complex and an inner medulla.2,6 When comparing HFUS with trichoscopy, it has been reported that the number of follicular units does not differ significantly, and only their width is greater with ultrasound measurements.7 In UHFUS, the hair tracts are observed inside the follicles before emerging to the surface.2,5
In androgenetic alopecia (AGA), hypoechogenicity of the involved hair follicles has been reported, a finding whose pathophysiology remains unclear and which could be attributed to inflammation.8 In a prospective study of 33 patients with AGA and 10 healthy controls,9 shallower follicles in the dermis and a replacement of the normal predominantly trilaminar pattern of hair shafts by a bilaminar one and areas of absence of hair follicles were found in AGA.
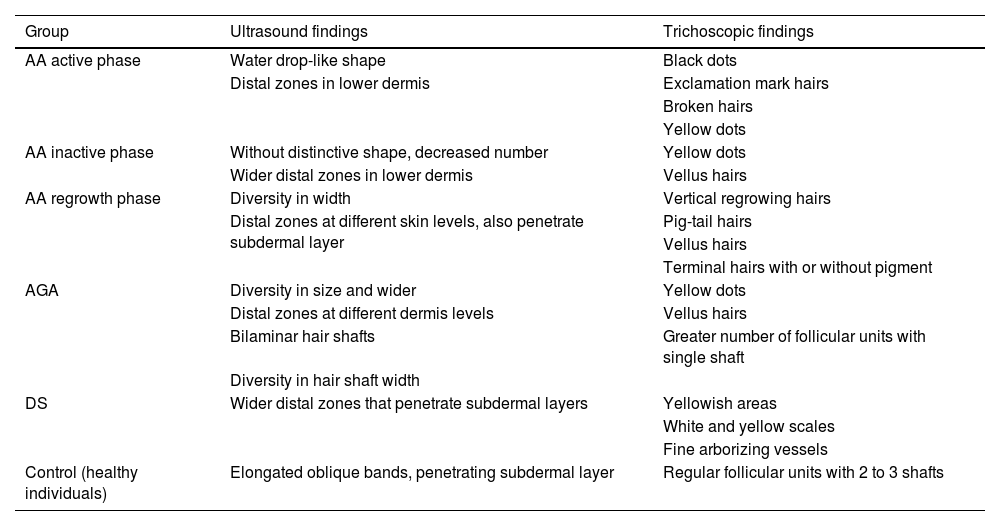
In alopecia areata (AA), signs such as empty hair follicles and perifollicular hyperechogenicity in the subcutaneous tissue8 have been identified, which could facilitate the direct evaluation of the severity of the disease and thus help design therapeutic strategies. In turn, the findings recently reported by Mikiel et al.10 are interesting, who characterized the ultrasound images of 25 patients with AA and its phases, 10 with AGA, and 12 with seborrheic dermatitis, correlating the findings with trichoscopy (Table 1). In active AA, clear and widened water drop-like follicles were described; the inactive phase was characterized by a smaller number of follicles without well-demarcated borders; and in the regrowth phase, follicles of different widths, elongated and with widened distal parts, were found. Both the width and echogenicity of the follicles in AA, regardless of the stage of the disease, were significantly greater. In cases of AGA, the width of the follicles was the smallest of all the groups studied.
Ultrasound findings of follicular structures (FS) vs trichoscopy in each studied group.
| Group | Ultrasound findings | Trichoscopic findings |
|---|---|---|
| AA active phase | Water drop-like shape | Black dots |
| Distal zones in lower dermis | Exclamation mark hairs | |
| Broken hairs | ||
| Yellow dots | ||
| AA inactive phase | Without distinctive shape, decreased number | Yellow dots |
| Wider distal zones in lower dermis | Vellus hairs | |
| AA regrowth phase | Diversity in width | Vertical regrowing hairs |
| Distal zones at different skin levels, also penetrate subdermal layer | Pig-tail hairs | |
| Vellus hairs | ||
| Terminal hairs with or without pigment | ||
| AGA | Diversity in size and wider | Yellow dots |
| Distal zones at different dermis levels | Vellus hairs | |
| Bilaminar hair shafts | Greater number of follicular units with single shaft | |
| Diversity in hair shaft width | ||
| DS | Wider distal zones that penetrate subdermal layers | Yellowish areas |
| White and yellow scales | ||
| Fine arborizing vessels | ||
| Control (healthy individuals) | Elongated oblique bands, penetrating subdermal layer | Regular follicular units with 2 to 3 shafts |
AA: alopecia areata; AGA: androgenetic alopecia; DS: seborrheic dermatitis.
The first publications that used HFUS in the evaluation of scarring alopecias showed that dermal inflammation appears hypoechoic and subcutaneous inflammation, hyperechoic, and, with Doppler, the affected areas are hypovascular,2,4,9,11 However, increased vascularization in the scarred areas using microvascular imaging modalities and even greater thickness and stiffness with shear wave elastography12 have also been reported.
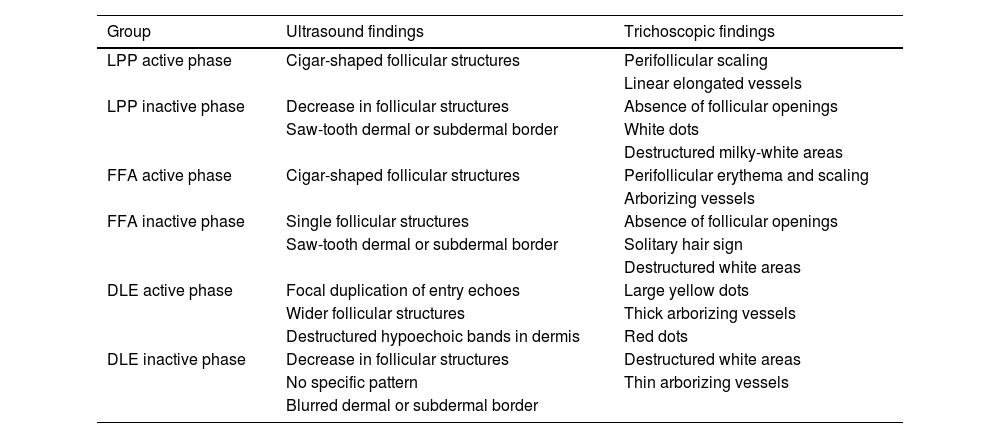
Recently, HFUS was evaluated as a diagnostic method in 8 patients with lichen planopilaris (LPP), 11 with discoid lupus erythematosus (DLE), 14 with frontal fibrosing alopecia (FFA), and 11 healthy volunteers.13 The active form of the disease in all groups was characterized by follicular structures with morphology and parameters different from those observed in the control group; the inactive forms were characterized by a significant reduction in the number of follicles (Table 2). The echogenicity of the skin and follicular structures was significantly higher than in healthy volunteers, which is likely due to the underlying fibrosis process. Of note, the absence of follicular openings in trichoscopy often did not correlate with the total lack of follicular structures in ultrasound images. All these findings indicate that HFUS allows a more precise evaluation of when the scarring process is complete.
Ultrasound characteristics of follicular structures and trichoscopic findings in scarring alopecias.
| Group | Ultrasound findings | Trichoscopic findings |
|---|---|---|
| LPP active phase | Cigar-shaped follicular structures | Perifollicular scaling |
| Linear elongated vessels | ||
| LPP inactive phase | Decrease in follicular structures | Absence of follicular openings |
| Saw-tooth dermal or subdermal border | White dots | |
| Destructured milky-white areas | ||
| FFA active phase | Cigar-shaped follicular structures | Perifollicular erythema and scaling |
| Arborizing vessels | ||
| FFA inactive phase | Single follicular structures | Absence of follicular openings |
| Saw-tooth dermal or subdermal border | Solitary hair sign | |
| Destructured white areas | ||
| DLE active phase | Focal duplication of entry echoes | Large yellow dots |
| Wider follicular structures | Thick arborizing vessels | |
| Destructured hypoechoic bands in dermis | Red dots | |
| DLE inactive phase | Decrease in follicular structures | Destructured white areas |
| No specific pattern | Thin arborizing vessels | |
| Blurred dermal or subdermal border |
FFA: frontal fibrosing alopecia; DLE: discoid lupus erythematosus; LPP: lichen planopilaris.
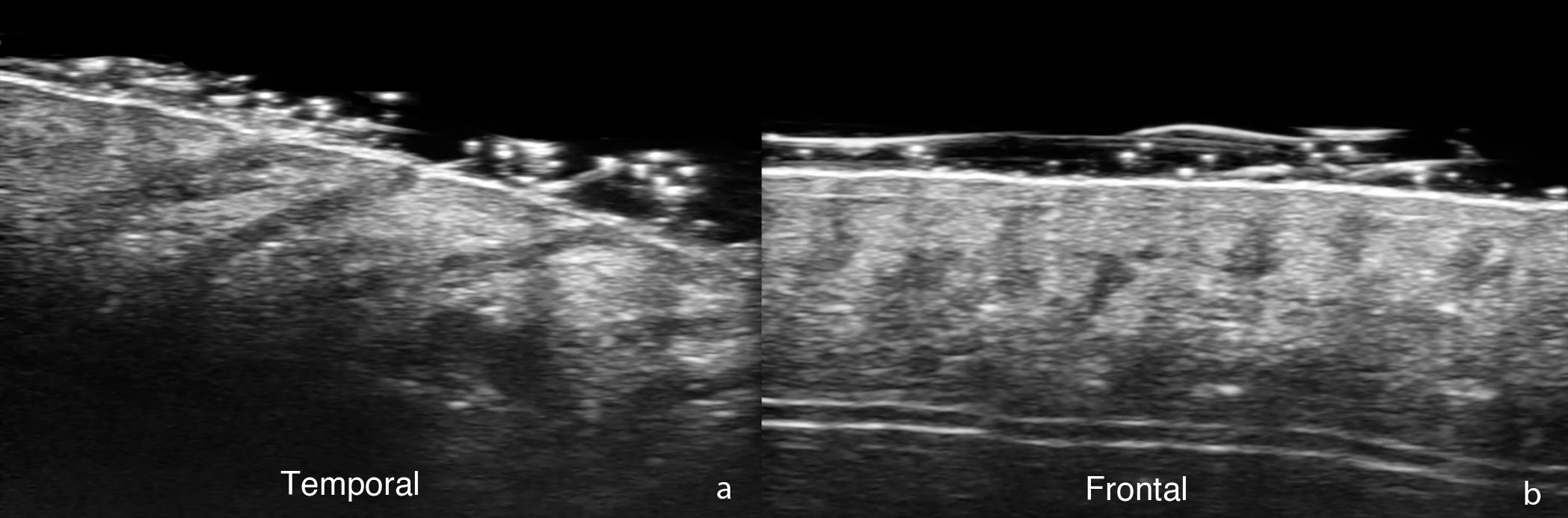
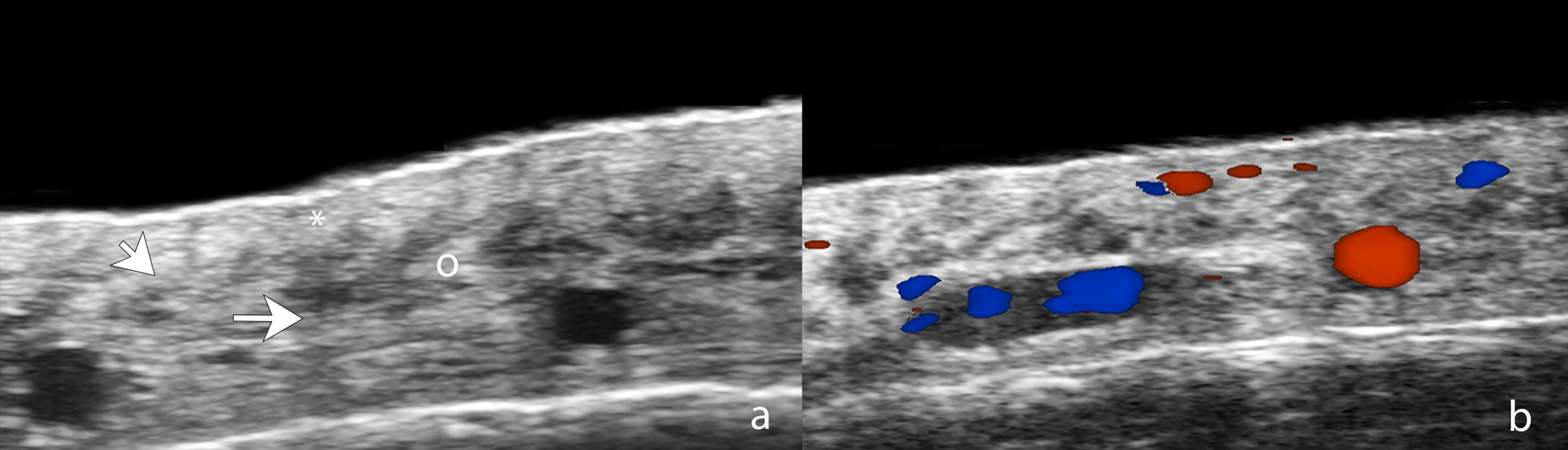
For its part, UHFUS in FFA has reported findings such as perifollicular hypoechogenicity in the mid-dermis, distal ambiguity of hair follicles, and undulating hyperechoic bands in the subcutaneous cellular tissue (Fig. 2). The first 2 were also observed in LPP and corresponded in histopathology to perifollicular fibrosis and inflammation in the promontory and to inflamed or destroyed follicles in the isthmus or infundibulum, respectively. In turn, the hyperechoic bands in the subcutaneous tissue corresponded to fibrous septa distributed linearly in the hypodermis.8 Another ultrasound study in 99 women with FFA and 40 controls showed greater vascular diameter and greater flow in the hairline area vs the alopecic band area and the control group, which can be explained by both active inflammation in the frontal line and fibrosis in the alopecic band. In addition, the diameter of the vessels was significantly greater in the healthy scalp area of patients with FFA vs the control group, which could be explained by subclinical inflammation and could be a predictor of future progression.14
Ultrasound of frontal fibrosing alopecia. (A) Loss of hair follicles (*) and subcutaneous adipose tissue. Loss of definition of the dermohypodermal border (oblique arrow), increased subcutaneous echogenicity (o), and prominent subcutaneous lamellar structures (horizontal arrow). (B) Color Doppler of the same region demonstrates increased dermal vascularization.
Dissecting cellulitis presents ultrasound findings similar to hidradenitis suppurativa and includes dilation of hair follicles, pseudocysts (<1cm), hypoechoic collections (>1cm), tunnels that run through the dermis and subcutaneous tissue and may present variable degrees of fibrosis and, with color Doppler, variable degrees of dermohypodermal hypervascularization in the periphery of the lesions.15
Reflectance confocal microscopyRCM is an optical imaging modality based on the emission of low-power laser light, which is reflected by structures depending on their different refractive indices. Images are obtained at different focal planes, allowing 3D analysis and visualization of cellular contours, as in histopathology. Its advantage is the high axial resolution, and its main limitation is its low penetration, as it only reaches depths of 250–350μm, visualizing only up to the superficial reticular dermis.16,17 DUE to the possibility of observing detailed microscopic changes, RCM is also being used in trichology and can be considered an intermediate step between trichoscopy and histology. With RCM, normal hair follicles have been described as structures of different sizes in an orderly pattern, with small ovoid or polygonal basal cells and larger and flatter central cells.3
Non-scarring alopeciaRudnicka et al.3 published a small case series in which RCM was used to characterize some hair diseases. The structure of the hair shaft presented the same appearance in healthy controls, AA, and AGA; however, heterogeneity in the thickness of the shafts was observed in the latter.
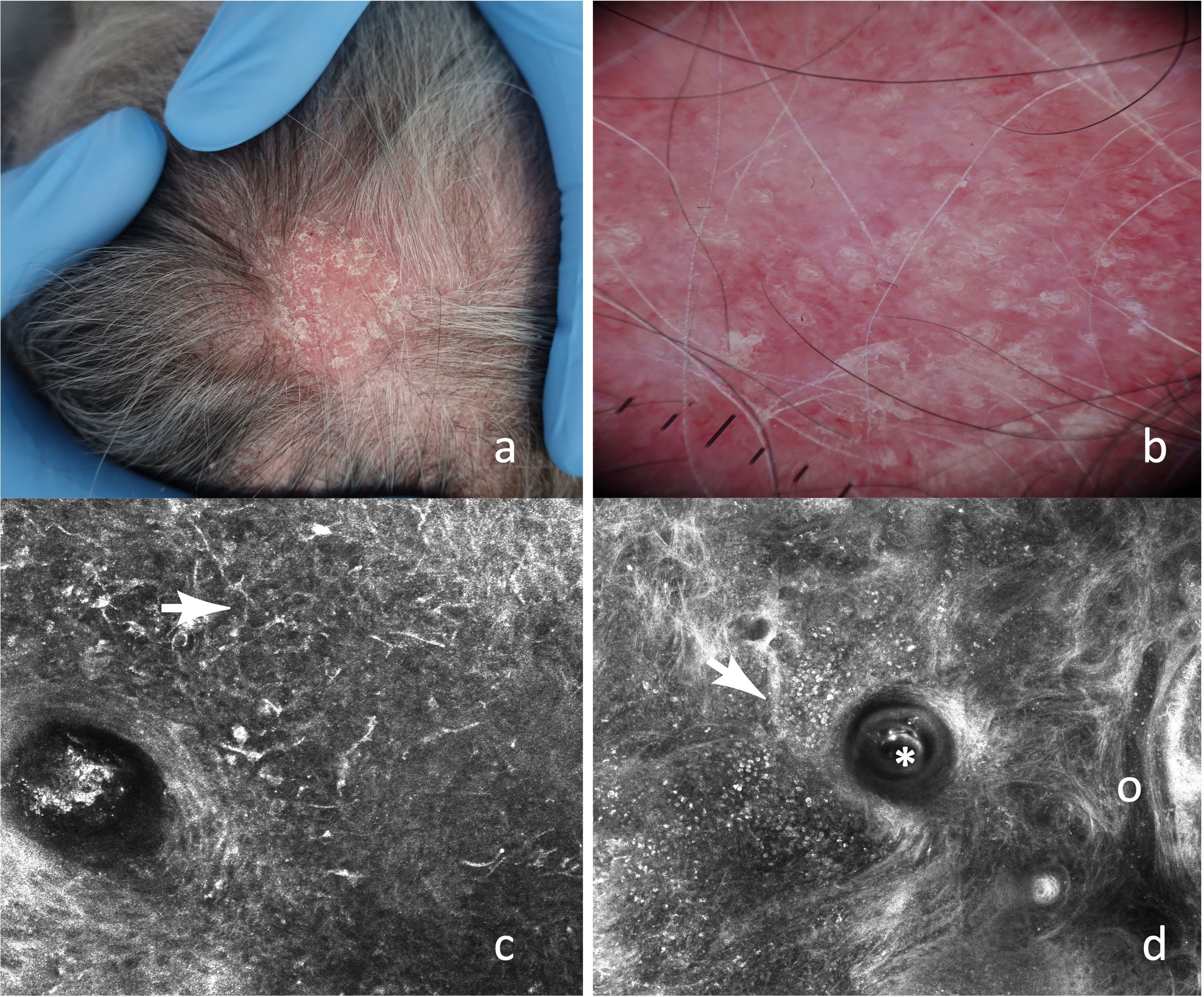
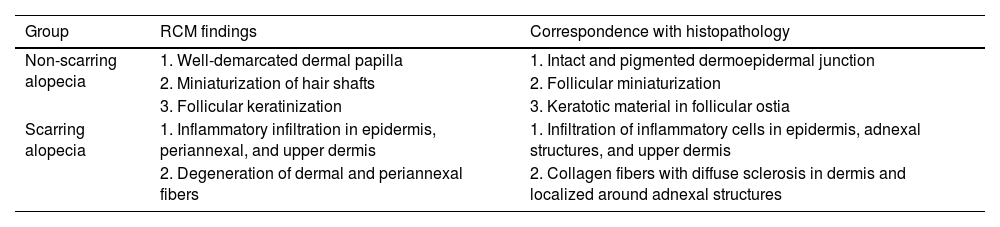
RCM criteria for the diagnosis of non-scarring alopecias such as delimited dermal papillae, miniaturized hair shafts, and follicular keratinization have been reported (Fig. 3). These findings were published in a 2016 retrospective study,18 in which several descriptive parameters of scarring (28 with LPP and 9 with DLE) and non-scarring alopecias (30 with AGA and 19 with AA) were established, and statistically significant characteristics for the 2 types of alopecia were identified (Table 3). Although no significant distinctive features were identified for AGA vs AA, inflammatory cells were found in the upper epidermis and around adnexal structures in several patients with AA, which could be explained by its autoimmune origin.
(A) Erythematous-desquamative plaque on the vertex. (B) Dermoscopy: diffuse erythema, follicular plugs, and fine arborizing vessels. (C) Epidermal RCM: dendritic-like cells with peri- and interfollicular distribution (
). (D) RCM at the DEJ: follicular plug (*), inflammatory infiltrate composed of round-shaped cells (), and dilated vessels (o).Distinctive RCM findings associated with alopecia diagnosis and their respective histopathology.
| Group | RCM findings | Correspondence with histopathology |
|---|---|---|
| Non-scarring alopecia | 1. Well-demarcated dermal papilla | 1. Intact and pigmented dermoepidermal junction |
| 2. Miniaturization of hair shafts | 2. Follicular miniaturization | |
| 3. Follicular keratinization | 3. Keratotic material in follicular ostia | |
| Scarring alopecia | 1. Inflammatory infiltration in epidermis, periannexal, and upper dermis | 1. Infiltration of inflammatory cells in epidermis, adnexal structures, and upper dermis |
| 2. Degeneration of dermal and periannexal fibers | 2. Collagen fibers with diffuse sclerosis in dermis and localized around adnexal structures |
In tinea capitis, homogeneous rounded hyperreflective structures of 5–10μm have been described in the proximal area of the hair shaft, corresponding to conidia that, in vivo, were observed compact, while ex vivo, they were more dispersed.19,20
For its part, distinctive findings have also been found in chemotherapy-induced alopecia. In a prospective observational study of 16 women with breast cancer, confocal and trichoscopic characteristics specific to the evolutionary phases of chemotherapy-induced alopecia were described.21
Scarring alopeciaWith RCM, inflammatory cells in the dermis, epidermis, and around adnexal structures have been described, which is consistent with the inflammatory or autoimmune pathogenesis of scarring alopecias. In turn, 3 highly specific characteristics have been identified: inflammatory cells in the epidermis, absence of miniaturization, and absence of follicular keratinization.18 In fact, one of the main criteria that allows discrimination between scarring and non-scarring alopecias is active inflammation in the adnexal epithelium or dermoepidermal junction, with the corresponding darkening of the dermal papillae and absence of their normal border. Another important criterion is dermal sclerosis, characterized by increased and thickened fibers arranged radially around the follicle. Although these findings are typical of scarring alopecias, they have been reported in some cases of chronic non-scarring alopecia, such as in the late phase of AGA, which is attributed to severe sun damage and chronic inflammation.18,22
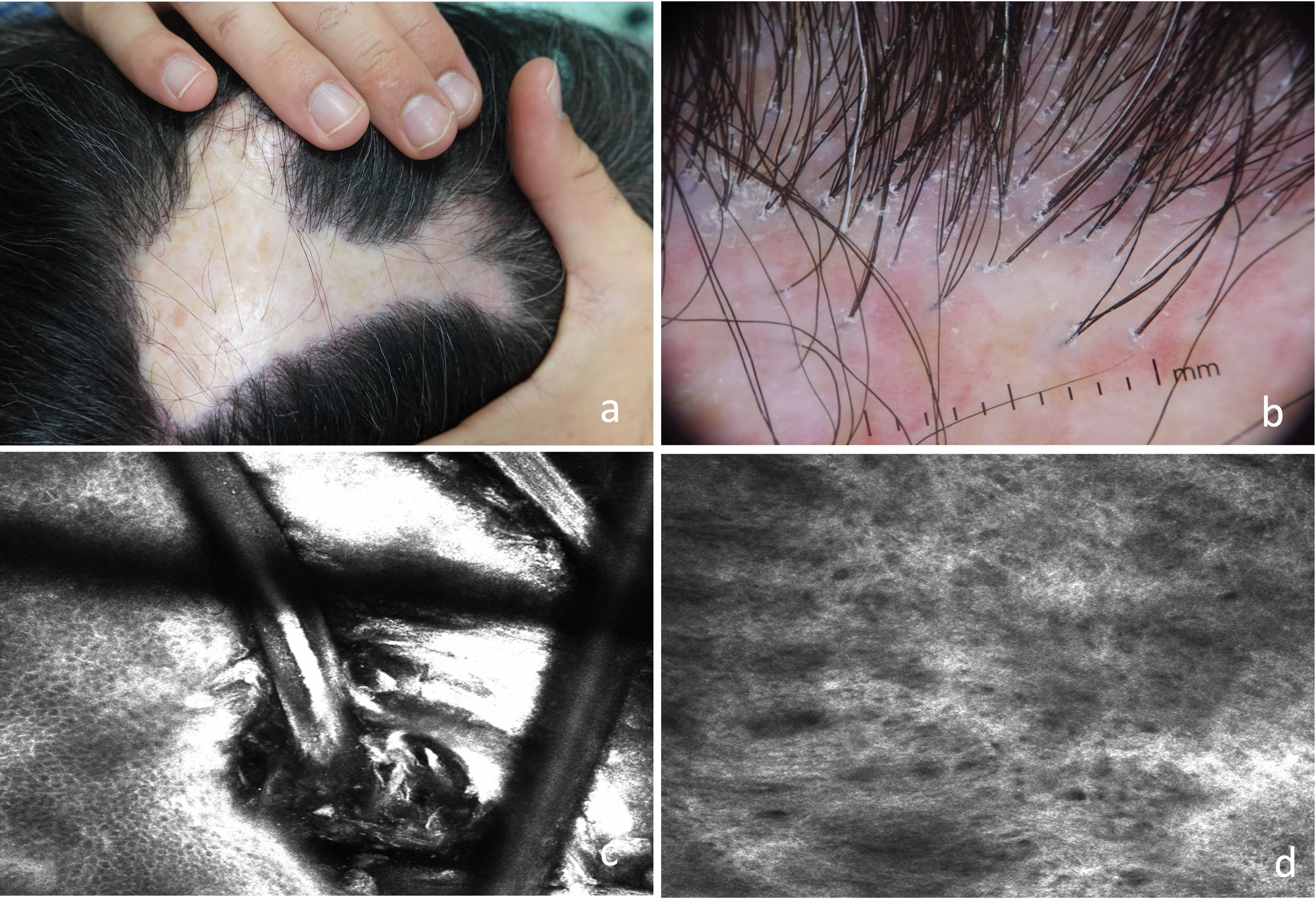
Regarding LPP and FFA, there is a specific study from 202023 in which 10 patients with LPP and 2 with FFA were evaluated, and inflammatory cells in the epidermis next to the adnexal infundibulum were described in almost half of the cases, a finding consistent with the histopathology of early stages.18,23 In long-standing lesions, dilated blood vessels, an increase in poorly defined thick dermal fibers, and extensive perifollicular fibrosis were observed (Fig. 4).
(A) Scarring alopecic plaque in the interparietal area. (B) Trichoscopy: erythema, perifollicular hyperkeratosis, and follicular tufting. (C) RCM: infundibular hyperkeratosis with whitish material filling the infundibulum of the hair follicle and surrounding the hair shaft. (D) Dermis: increased number of poorly defined and thick dermal fibers.
In LPP and DLE, characteristics that have shown correlation with histopathology have also been described. There is a predominance of inflammatory infiltrate in the epidermis and diffuse interface changes in LPP, focal changes in DLE with dermoepidermal junction involvement, and changes located exclusively in the basal layer of the adnexal epithelium in LPP with prominent follicular involvement. These findings were described in a study of 7 patients with a previous histological diagnosis of LPP and DLE.24
Particularly for cases of DLE, the diagnostic process can be difficult, so the use of RCM is promising. An observational study of 12 patients with DLE found that interface dermatitis and infundibular dilation were the characteristics that exhibited the highest level of agreement with histopathology.25 In turn, confocal analysis associated with 3 trichoscopic findings managed to improve the agreement with histopathology.
Morphea and folliculitis decalvans have also been reported. In a patient diagnosed with coup de saber morphea, RCM showed marked hyperreflective areas with severe atrophy of eccrine glands, disappearance of sebaceous glands, and a few small follicles,26 which contributed to the diagnosis of early (with a predominance of lymphocytic infiltrate) and late stages (with more prominent sclerosis). Regarding folliculitis decalvans, RCM in 14 patients identified highly reflective tubular structures, bright round or oval structures surrounded by a regular border, and interfollicular hyperkeratosis, corresponding in trichoscopy to tufted hairs, pustules, and scales or crusts, respectively.27,28
Optical coherence tomographyOCT is an imaging modality that uses broadband near-infrared light to detect backscattered photons at different depths and, with this, creates high-resolution cross-sectional images. Its use has spread in dermatology as it allows visualization of cellular and morphological changes up to 2–3mm deep.29 In trichology, it has recently been incorporated as a diagnostic tool. It shows the scalp as a bright surface and hair shafts as dark and hollow lumens within which there are bright and reflective rings.4 Its advantage is the high resolution, similar to that of UHFUS, and its main limitation is the low penetration, as it does not allow observation of the complete subcutaneous cellular tissue.
Non-scarring alopeciasTo determine hair morphology and vitality with OCT, 2 parameters are used that allow comparisons and analyses in studies: cross-section (CS) and form factor (FF), the latter determined by the ratio of the maximum and minimum diameters of the hair. The rate of variation in CS in healthy volunteers is reportedly<3%, while the corresponding FF varies by ≤4%30 and has been observed to worsen in patients undergoing chemotherapy31 and with AA.32
The examination of 20 hairs from the frontal and occipital areas, ex vivo, in women with breast cancer on tamoxifen (17) vs chemotherapy (17) showed that the mean diameter after chemotherapy was 15% lower and the CS was significantly smaller than in the hairs obtained prior to therapy; although the FF did not vary for the frontal area, it did in the occipital area. No changes in CS or FF were observed in patients on tamoxifen.
García Bartels et al.32 published morphological changes detected with OCT in 9 patients with AA. The CS in the alopecic patches was significantly smaller vs unaffected areas; however, the FF did not show significant differences. These results indicate that structural abnormalities of the shafts are found only in active AA lesions. Another publication monitored the progression of 2 patients with patchy AA and 1 with universal AA treated with 3 sessions of platelet-rich plasma33. They found an increase in the length and number of follicles in patchy AA and no significant changes in the patient with universal AA. A different study on AA found that 22 out of 30 scans of the dermoepidermal junction had more follicles than on the surface,4 indicating the presence of growing hairs in AA that are not visible on the surface.
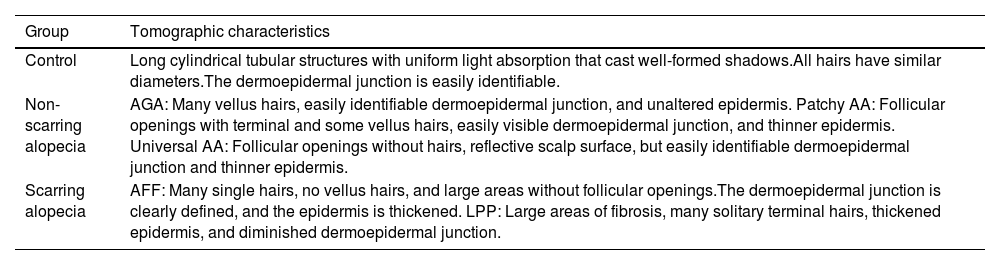
Scarring alopeciasA recent publication proposes an atlas of tomographic characteristics obtained from the evaluation of 6 healthy subjects, 12 with scarring alopecia, and 14 with non-scarring alopecia.34 They highlight the findings of increased epidermal thickness and decreased follicle count in the scarring alopecia group vs the non-scarring alopecia and control groups. Although few patients are analyzed, this study provides a valuable set of images potentially useful for the monitoring and management of alopecias, including scarring alopecias (Table 4).
Main findings with OCT in the studied groups.
| Group | Tomographic characteristics |
|---|---|
| Control | Long cylindrical tubular structures with uniform light absorption that cast well-formed shadows.All hairs have similar diameters.The dermoepidermal junction is easily identifiable. |
| Non-scarring alopecia | AGA: Many vellus hairs, easily identifiable dermoepidermal junction, and unaltered epidermis. Patchy AA: Follicular openings with terminal and some vellus hairs, easily visible dermoepidermal junction, and thinner epidermis. Universal AA: Follicular openings without hairs, reflective scalp surface, but easily identifiable dermoepidermal junction and thinner epidermis. |
| Scarring alopecia | AFF: Many single hairs, no vellus hairs, and large areas without follicular openings.The dermoepidermal junction is clearly defined, and the epidermis is thickened. LPP: Large areas of fibrosis, many solitary terminal hairs, thickened epidermis, and diminished dermoepidermal junction. |
In FFA, OCT has been used to show that epidermal thickness is greater at the hairline and smaller in the scarring alopecic band.35 These findings are explained by the presence of inflammatory infiltrate and edema in areas of activity and atrophy in the scarred areas, respectively. In addition, as the inflamed areas progress to scarred areas, the vascular flow of the superficial plexus progressively decreases, and the deeper flow increases, which would occur due to obliteration of superficial vessels in the scarring stage with redistribution of flow toward the deeper ones.
ConclusionsAlthough the gold standard in the evaluation of hair diseases is histological analysis, it constitutes an invasive modality and is not always representative of the overall process. In turn, trichoscopy provides an enlarged image of the surface, hair shafts, and follicular openings, but the underlying follicles are inaccessible. Therefore, these new non-invasive diagnostic tools provide more information and complement traditional methods by evaluating deeper structures and even allowing their analysis during and after implementing a treatment. However, there are still several difficulties in adopting the use of all these techniques in trichology, such as high costs, learning curve for the dermatologist, inherent difficulty of the anatomical area, studies with a small number of patients, and the absence of comparison of histopathological and trichoscopic images. Additionally, the advantages and limitations of each imaging modality should be taken into consideration to adequately leverage the anatomical information provided by each modality.
Conflicts of interestNone declared.