A 50-year-old woman with no relevant dermatologic history was diagnosed with a retroareolar right breast infiltrating ductal carcinoma, stage T2N3M1, grade II, hormone-receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2−), with a Ki-67 proliferation index of 40%. Staging studies showed multiple hepatic metastases and a bone metastasis in the right lateral 8th rib. The Oncology Department initiated treatment with leuprorelin, letrozole, and ribociclib. The patient exhibited a good response and adequate treatment tolerance. However, 14 months after treatment initiation, she developed hypopigmented lesions on the face and neck, for which she was referred to Dermatology for evaluation.
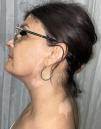
Physical examinationExamination revealed acral macules with well-defined borders located on the posterior neck, as well as periocular, frontal, and preauricular regions of the face (Figs. 1 and 2). The lesions became more evident under Wood's lamp examination (Fig. 3).
Additional testsA blood test, including thyroid function profile and antithyroid antibodies, showed no abnormalities.
What is your diagnosis?
DiagnosisRibociclib-induced vitiligo.
Disease progression and treatmentSince stable oncologic response achieved, ribociclib was continued. Topical tacrolimus ointment and controlled sun exposure were initiated, with partial improvement.
CommentaryVitiligo is a multifactorial acquired autoimmune disease characterized by circumscribed depigmented macules and patches due to selective melanocyte destruction.1–3 Its etiology is unknown, and in some cases it has been associated with different drugs, especially tumor necrosis factor inhibitors (anti-TNF), immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors), and more recently, cyclin-dependent kinase (CDK) inhibitors.4 CDKs are a large family of kinases essential for cell-cycle regulation. CDK4/6 inhibitors selectively block CDK4 and CDK6, preventing CDK4/6-mediated phosphorylation of the retinoblastoma (Rb) protein. As a result, the cell cycle is arrested between the G1 and S phases, halting cell division.1–3 The 3 CDK4/6 inhibitors approved by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) are ribociclib, palbociclib, and abemaciclib. Along with hormone therapy, these agents constitute first-line treatment for HR+/HER2− locally advanced or metastatic breast cancer – the most common subtype of breast cancer.1–3,5,6 The most frequent adverse effects include hematologic abnormalities (neutropenia and leukopenia), GI symptoms (nausea, vomiting, diarrhea), and fatigue. Cutaneous adverse effects account for approximately 15% of cases, the most common being alopecia, pruritus, and morbilliform eruptions.1–3,5,6 Most are mild, but severe reactions such as toxic epidermal necrolysis, Stevens–Johnson syndrome, and acute generalized exanthematous pustulosis have been described. Other less frequent but more specific dermatologic adverse events include vitiligo and cutaneous lupus erythematosus.2 Recently, cases of vitiligo have been reported primarily in association with ribociclib therapy. The mean time to onset is approximately 9 months.2 Exacerbations in patients with pre-existing vitiligo have also been described. Lesions may appear in localized areas – particularly photo-exposed sites – or may be generalized. Most patients show involvement of >25% of the body surface area. Regarding etiopathogenesis, it has been hypothesized that apoptosis induced by CDK4/6 pathway inhibition may lead to widespread melanocyte apoptosis.1–3,5,6 The prognostic significance of vitiligo in these patients remains unclear and is generally not associated with an improved oncologic response. Even after treatment discontinuation, lesions may persist. Management is challenging and may include topical corticosteroids, topical tacrolimus, narrow-band UVB phototherapy, or oral corticosteroids.1–3,5,6