Among the different approaches for improving the effectiveness in the treatment of Capillary Malformations type Port Wine Stain (CM type PWS) are the intense pulsed light sources. There are few clinical studies prove useful in the treatment of CM. Furthermore, no studies have been published yet demonstrating the histological effects of IPL in CM.
ObjectivesTo assess the histological effects of pulsed light in capillary malformations type port wine stain. We wanted to compare epidermal, dermal and vessel wall damage after treatment with different combinations of IPL parameters.
Material and methodsFifty-five post-treatment biopsies were performed in 15 consenting patients with CM and stained with nitroblue-tetrazolium chloride (NBTC). Patients had not been treated previously.
ResultsFifteen patients with CM, with a median age of 39 years-old were enrolled in this study. In this series, the patients with the most severe epidermal damage were those with a darker phototype. Pink CM were especially resistant to treatment, even using high fluences, short pulse durations and stacking pulses. Longer intra- and interpulse delays were effective in purple CM, achieving adequate vessel destruction.
ConclusionsIPL devices provide a vast amount of treatment possibilities and further studies are necessary to optimize therapeutic approaches to CM. In this study we have observed the histological effects of different pulses on the MC type PWS.
Entre las distintas estrategias para intentar mejorar la eficacia en el tratamiento de las malformaciones capilares tipo mancha en vino de Oporto (MC tipo MVO) están las fuentes de luz pulsada intensa. Existen hasta la fecha pocos estudios clínicos que avalen su utilidad en el tratamiento de las MC. Además, no disponemos de estudios histológicos que objetiven los efectos de la luz pulsada en la coagulación de estos vasos anómalos.
ObjetivosEvaluar los efectos histológicos de la luz pulsada en las MC tipo MVO. Intentamos comparar el daño epidérmico, dérmico y de la pared de los vasos después del tratamiento con distintos parámetros de IPL.
Material y métodosFueron realizadas 55 biopsias postratamiento en las MC de 15 pacientes. Las muestras fueron teñidas con cloruro de nitroblue tetrazolium.
ResultadosQuince pacientes (edad media: 39 años) fueron inscritos en este estudio. En esta serie los pacientes con mayor daño epidérmico fueron aquellos con un fototipo más alto (>iv). Las malformaciones de color rosa pálido eran especialmente resistentes al tratamiento, incluso con altas fluencias, duraciones de pulso corto y pulsos repetidos. Los pulsos de una mayor duración fueron especialmente eficaces en malformaciones capilares violáceas.
ConclusionesLos equipos de IPL ofrecen una gran cantidad de opciones de tratamiento en las MC, sin embargo necesitamos conocer mejor sus efectos para realizar abordajes más eficaces y seguros. En este estudio hemos podido observar los efectos histológicos de los distintos pulsos sobre las MC tipo MVO.
Capillary malformations type port wine stain (CM type PWS) constitute congenital anomalies that are believed to represent errors in vascular development during embryogenesis.1–4
Currently, pulsed dye laser (PDL) remains the gold standard treatment for CM.1,4–6 However, 25–50% of treated lesions do not demonstrate significant improvement.7 Other options have been used in resistant lesions, such as alexandrite, neodymium-YAG, dual wavelength lasers8 and, more recently intense pulsed light (IPL) sources.3
IPL systems emit non-coherent broadband light with wavelengths in the 515–1200nm range6 and, theoretically, this spectrum matches the absorption coefficient and thermal relaxation time of a broader range of vessels within CM.9 Similar to laser systems (though less selective than them), this technology seems to respect the principle of selective photothermolysis (SP),3,10 which consists in the preferential absorption of light by oxy/deoxy-hemoglobin and the subsequent conversion into thermal energy, leading to the selective coagulation of blood vessels.11 Specific output wavelengths depend on the cutoff filters used,6 which optimize absorption of the target chromophores, reduce the strong absorption of melanin and prevent adverse effects such as erythema, blistering and crusting.12 Recently, the optimized pulsed light sources (OPL) have been developed, providing a dual-band output spectrum from 500 to 670nm and 870 to 1200nm, which are even more selective to oxy/deoxy-hemoglobin. Theoretically, the use of OPL reduces the risk of epidermal damage, by displacing the interval between 610 and 870nm, characterized by melanin's absorption peak.13
IPL systems allow the individual selection of multiple parameters, such as: wavelength, pulse duration, fluence, multipulse mode and intrapulse time delay.12 On the other hand, we can also use only one pulse or multiple pulses, with different delays, creating a multiplicity of possible combinations.
Although IPL sources have been increasingly used in the treatment of CM, there are very few clinical studies regarding their effectiveness.5,11 Furthermore, we have not found any published histochemical studies describing the histological effects of IPL treatment of CM. We aim to describe the vascular, epidermal and dermal tissue damage in CM treated with IPL with variations in multiple parameters using nitroblue-tetrazolium chloride (NBTC) histochemical staining of biopsies taken from the CM immediately after treatment.4,14–17
Material and methodsFifteen adult patients with CM were enrolled in this study. Four men and eleven women were treated with the Ellipse Flex (Ellipse®, Denmark) IPL device that can provide two different spectrums of polychromatic light, according to the different cut-off filters used: between 555 and 950nm (VL-2®) and 530 and 750nm (PR®). For epidermal protection, a cold-air cooling system (Cryos5™, Zimmer Medizinsysteme GMbH, Neu-Ulm, Germany) and an ultrasound gel were used. Different wavelengths, fluences, pulse durations, inter- and intradelay times with stacking pulses18,19 and multiple passes were used to treat an average of four CM test areas per patient (range 2–7). The hospital's ethics committee approved the study. Fully informed written consent was obtained from all patients before the first treatment. A technical description of the IPL device and the parameters used in the study are shown in Table 1.
Characteristics of the IPL device and the parameters used.
| Technical characteristics | |
|---|---|
| Wavelength/emission spectrum | 555–950nm 530–750nm |
| Light characteristics | Polychromatic, incoherent |
| Shape of spot | Rectangular |
| Spot size (cm2) | 5.0 (10×48mm) |
| Cooling | Cold-air cooling and ultrasound gel |
| Pulse duration (ms) | 2.5–8 |
| Fluence range (J/cm2) | 5.3–19.4 |
| Number of minipulses in one pulse | 1–4 |
| Number of pulses | 1–7 |
| Delay (inter-/intrapulses) | 1a–60s/1.5– 200ms |
Post-treatment biopsies of each of the 55 test areas were performed, 10min after a single IPL treatment using a 3-mm punch. We chose slightly sun-exposed skin areas in order to minimize epidermal damage and hair-free areas to avoid melanin light absorption, since melanin behaves as a competitive chromophore to oxyhemoglobin.9,20 Biopsies were frozen in liquid nitrogen and the tissue sections were stained with NBTC, as described in previous studies.4,14,16 This enzyme is a redox indicator that is reduced by NADPH-diaphorase. This enzymatic activity stops immediately after cell death and is present in endothelial cells, fibroblasts and smooth muscle fibers but not in the dermal connective matrix. Therefore, only the viable tissue stains blue, allowing an easy differentiation from thermally damaged tissue.
Three parameters were evaluated: vessel wall destruction, epidermal and dermal damage, using a semiquantitative score: 0, absent; 1, minimal; 2, moderate; and 3, extensive tissue damage. Two dermatologists and one dermatopathologist evaluated all slides blindly. We always chose the majority agreement among the three observers, and in case of total disagreement, we suited the particular case to achieve a consensus agreement.
ResultsThe median age of the 15 patients was 39 years-old (21–80 years) and most biopsies were taken from the retroauricular area (47%) and back (33%). The areas within the treated CM were all flat, their color varied from pink to purple and the vessels had varying diameters (Table 2). The histochemical findings are illustrated in Table 3, showing how variations in each IPL parameter can modify the capacity of vessel wall destruction and production of epidermal and dermal collateral damage.
Characteristics of the patients and capillary malformations treated.
| Patients | Capillary malformations | |||||
|---|---|---|---|---|---|---|
| Sex | Age | Location | Color | Vessels caliber | Phototypes (Fitzpatrick) | |
| 1 | Female | 53 | Shoulder | 3 | 2 | III |
| 2 | Female | 45 | Back | 2 | 2 | IV |
| 3 | Female | 80 | Retroauricular | 3 | 2 | III |
| 4 | Female | 29 | Arm | 1 | 1 | II |
| 5 | Male | 27 | Retroauricular | 3 | 2 | IV |
| 6 | Male | 42 | Retroauricular | 2 | 2 | III |
| 7 | Female | 38 | Face | 2 | 2 | IV |
| 8 | Female | 38 | Retroauricular | 2 | 2 | II |
| 9 | Male | 59 | Back | 3 | 3 | III |
| 10 | Female | 57 | Retroauricular | 2 | 2 | III |
| 11 | Female | 54 | Back | 3 | 2 | II |
| 12 | Female | 31 | Back | 2 | 1 | II |
| 13 | Female | 21 | Retroauricular | 2 | 2 | II |
| 14 | Female | 39 | Back | 2 | 1 | III |
| 15 | Male | 38 | Back | 1 | 1 | III |
*Color of capillary malformation (colorimetric scale)28: pink (1); red-violaceous (2) or purple (3).
**Vessel caliber: thin 10–50μm (1); medium 50–200μm (2) or large ≥200μm (3).
IPL parameters used and histopathological findings seen with the NTBC histochemical stained biopsies.
| Patients | IPL Parameters | Histopathological findings | ||||||
|---|---|---|---|---|---|---|---|---|
| Wavelengths (nm) | Pulsesa (number) | Fluence (J/cm2) | Pulse duration (ms) | Delay time: inter-/intrapulses | Vessel wall damage | Epidermal damage | Dermal damage | |
| 1 | 555–950 | 1 | 19 | 8 | –/– | 3 | 0 | 2 |
| 555–950 | 2 | 19 | 8 | 60s/– | 3 | 0 | 2 | |
| 530–750 | 1 | 8.9 | 8 | –/– | 2 | 0 | 0 | |
| 530–750 | 1 | 5.3 | 2.5 | –/– | 1 | 1 | 0 | |
| 555–950 | 1 | 7.9 | 2.5 | –/– | 1 | 0 | 0 | |
| 2 | 555–950 | 1 (3 mp) | 19.4 | 2.5 | –/200ms | 1 | 2 | 0 |
| 555–950 | 1 (3 mp) | 19.4 | 2.5 | –/100ms | 0 | 3 | 0 | |
| 555–950 | 1 (3 mp) | 19.4 | 2.5 | –/50ms | 0 | 3 | 0 | |
| 3 | 555–950 | 1 | 15 | 8 | –/– | 2 | 1 | 1 |
| 555–950 | 2 | 15 | 8 | 1s/– | 3 | 2 | 3 | |
| 555–950 | 2 | 15 | 8 | 30s/– | 3 | 1 | 1 | |
| 555–950 | 1 | 13 | 8 | –/– | 1 | 2 | 0 | |
| 555–950 | 2 | 13 | 8 | 1s/– | 2 | 3 | 1 | |
| 555–950 | 2 | 13 | 8 | 30s/– | 1 | 3 | 1 | |
| 4 | 530–750 | 1 (3 mp) | 9 | 2.5 | –/1.5ms | 0 | 0 | 0 |
| 530–750 | 7 (3 mp) | 9 | 2.5 | 1s/1.5ms | 0 | 0 | 0 | |
| 5 | 555–950 | 1 | 19 | 8 | –/– | 3 | 2 | 1 |
| 555–950 | 2 | 19 | 8 | 60s/– | 3 | 3 | 3 | |
| 555–950 | 1 | 14 | 8 | –/– | 2 | 2 | 1 | |
| 555–950 | 2 | 14 | 8 | 60s/– | 2 | 3 | 1 | |
| 6 | 555–950 | 1 (4 mp) | 19.3 | 2.5 | –/1.5ms | 2 | 2 | 0 |
| 555–950 | 1 (4 mp) | 19.3 | 2.5 | –/200ms | 1 | 1 | 0 | |
| 7 | 555–950 | 1 | 19 | 8 | –/– | 2 | 3 | 2 |
| 555–950 | 2 | 19 | 8 | 1s/– | 3 | 3 | 3 | |
| 555–950 | 2 | 19 | 8 | 60s/– | 2 | 3 | 2 | |
| 555–950 | 1 | 13 | 8 | –/– | 1 | 3 | 0 | |
| 555–950 | 2 | 11 | 8 | 60s/– | 1 | 3 | 0 | |
| 8 | 530–750 | 1 | 5.3 | 2.5 | –/– | 1 | 0 | 0 |
| 555–950 | 1 | 7.9 | 2.5 | –/– | 1 | 0 | 0 | |
| 555–950 | 1 (3 mp) | 19.4 | 2.5 | –/50ms | 2 | 1 | 1 | |
| 555–950 | 1 (3 mp) | 19.4 | 2.5 | –/200ms | 1 | 1 | 1 | |
| 555–950 | 3 | 7.9 | 2.5 | 1s/– | 2 | 0 | 0 | |
| 555–950 | 1 (2 mp) | 13.4 | 2.5 | –/5ms | 3 | 0 | 0 | |
| 555–950 | 1 (3 mp) | 19.4 | 2.5 | –/5ms | 3 | 1 | 1 | |
| 9 | 555–950 | 1 (3 mp) | 19.3 | 2.5 | –/1.5ms | 3 | 3 | 3 |
| 555–950 | 1 (3 mp) | 19.3 | 2.5 | –/200ms | 3 | 2 | 2 | |
| 10 | 555–950 | 1 | 19 | 8 | –/– | 3 | 0 | 2 |
| 555–950 | 2 | 19 | 8 | 60s/– | 3 | 2 | 2 | |
| 555–950 | 1 | 16 | 8 | –/– | 2 | 1 | 1 | |
| 555–950 | 2 | 16 | 8 | 60s/– | 3 | 2 | 2 | |
| 11 | 555–950 | 1 (4 mp) | 19.3 | 2.5 | –/1.5ms | 3 | 1 | 2 |
| 555–950 | 1 (4 mp) | 19.3 | 2.5 | –/200ms | 3 | 0 | 1 | |
| 12 | 555–950 | 1 | 19 | 8 | –/– | 1 | 0 | 0 |
| 555–950 | 2 | 19 | 8 | 1s/– | 3 | 1 | 1 | |
| 555–950 | 2 | 19 | 8 | 60s/– | 3 | 1 | 1 | |
| 13 | 555–950 | 1 (4 mp) | 17.5 | 2.5 | –/5ms | 3 | 0 | 1 |
| 555–950 | 2 (4 mp) | 17.5 | 2.5 | 1s/5ms | 3 | 2 | 3 | |
| 555–950 | 1 (4 mp) | 17.5 | 2.5 | –/200ms | 1 | 0 | 0 | |
| 14 | 555–950 | 1 | 14 | 8 | –/– | 2 | 0 | 1 |
| 555–950 | 1 | 19 | 8 | –/– | 3 | 2 | 2 | |
| 555–950 | 2 | 19 | 8 | 60s/– | 3 | 2 | 3 | |
| 530–750 | 1 | 9.9 | 8 | –/– | 2 | 0 | 0 | |
| 15 | 555–950 | 1 (3 mp) | 19.2 | 2.5 | –/2.5ms | 0 | 0 | 0 |
| 555–950 | 2 (3 mp) | 19.2 | 2.5 | 1s/2.5ms | 2 | 2 | 0 | |
| 555–950 | 3 (3 mp) | 19.2 | 2.5 | 1s/2.5ms | 2 | 3 | 0 | |
1s, Interpulse delays of 1s correspond to stacking pulses; mp, minipulses. The histopathological findings are described using a semiquantitative scale (0–3), regarding: vessel wall damage (0, absent; 1, focal intravascular coagulation; 2, moderate destruction of vessel walls; 3, intense destruction of vessel walls), epidermal damage (0, absent; 1, focal epidermal damage; 2, moderate epidermal damage; 3, full epidermal necrosis) and dermal damage (0, absent; 1, focal dermal damage with perivascular collagen denaturation; 2, moderate dermal damage with a limited coagulation area; 3, intense dermal damage with an extensive coagulation area).
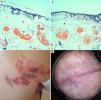
In this series, the patients with the most severe epidermal damage were those with a darker phototype (patients 2, 5 and 7). In both cases, in spite of intense epidermal cooling, severe epidermal damage was noted with high fluences (around 19J/cm2), but also with lower fluences (11 and 13J/cm2) and a long pulse duration (8ms) in case 7. Indeed, a significant proportion of the applied energy was absorbed by melanin in the basal layer of the epidermis, and the amount of energy that reached the dermis was insufficient to achieve enough heat to cause vessel wall destruction (Fig. 1).
In patients with a darker phototype, severe epidermal damage with full epidermal necrosis (§) was noted with (1a) high fluences (19.4J/cm2) in patient 2 and (1b) lower fluences: 13J/cm2 in patient 7. In these patients, focal intravascular coagulation was also seen (¥). No dermal damage was observed.
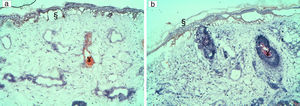
Patients with pink lesions were difficult to treat, as expected. In case 15, stacking a multipulse train of short pulses with high fluences obtained more vessel wall damage but with intense epidermal (but not dermal) damage. In case 4, using the shorter pulse duration (2.5ms) and a total energy of 9J/cm2 per pulse, even with seven stacking pulses, we could not obtain the necessary heating to cause any vascular or tissular alteration (Fig. 2). However, in case 12 both multiple and stacking pulses obtained more intense vessel wall damage than using only one pulse, without significant collateral damage.
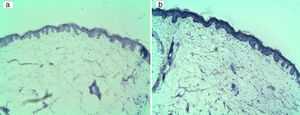
On the other hand, in a purple CM lesion treated with a fluence of 15J/cm2 and a pulse duration of 8ms, we found complete vessel wall destruction with less dermo-epidermal damage using multiple passes (interdelay time: 30s) instead of stacking pulses (case 3).
In some patients, using multiple pulses instead of a single pulse, improved treatment efficacy in CM with thin vessels. This happened in case 12 (fluence of 19J/cm2 and pulse duration of 8ms) and case 15 (fluence of 19.2J/cm2 and pulse duration of 2.5ms), where two pulses achieved a selective destruction of the vessel wall. In case 15, when we used 3 pulses (with the same referred parameters), the same level of vessel walls destruction was obtained (as with two pulses), but with more dermo-epidermal damage.
Intrapulse delay adjustments may also become extremely important to achieve complete destruction of vessel walls, with less collateral damage. Decreasing the intrapulse delay time, we obtained more efficient vessel walls destruction, without substantial increase of the dermo-epidermal damage (cases 8 and 13). Both patients had CM with medium caliber vessels. They were treated with high fluences (19.4J/cm2 in case 8 and 17.5J/cm2 in case 13) and intrapulse delay times were reduced from 50 to 5ms (case 8) and from 200 to 5ms (case 13). In case 8, however, an even more selective result was acquired, maintaining the referred parameters (including the 5ms intrapulse delay) and decreasing the total fluence (from 19.4 to 13.4J/cm2), without any dermo-epidermal damage.
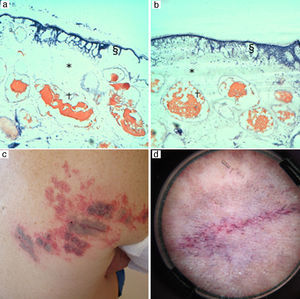
In some purple CM containing vessels with a medium to large vessel caliber, increased intrapulse delay times led to complete destruction of vessel walls, with less collateral damage. In cases 9 and 11, for example, increasing intrapulse delay times from 1.5 to 200ms resulted in complete vessel wall destruction with slightly less epidermal and dermal tissue damage (Fig. 3).
A purple CM (patient 9) was treated with a fluence of 19.3J/cm2 and a pulse duration of 2.5ms with delay times of (a) 1.5ms and (3b) 200ms. Intense destruction of the vessel walls (†) was achieved with both delay times. The shorter delay time (2a) lead to intense epidermal (§) and dermal (*) tissue damage in comparison to the moderate epidermal and dermal damage observed with the longer delay time (b). (c) Note the permanent gray in the central area (*) following treatment with high fluences (19.3J/cm2) and short pulse (2.5ms). (d) In dermoscopy we observe intense vessel coagulation.
In patients 1, 4, 8 and 14, who were treated with the PR® handpiece (530–750nm), the settings used seemed insufficient to destroy the vessel walls effectively, even when using a shorter pulse duration (2.5ms) with the highest fluences allowed by the device. As mentioned above, no vascular damage was observed in patient 4, even when stacking pulses.
DiscussionAlthough PDL is still the first choice for the treatment of CM, second generation IPL devices have improved their selectivity and have been increasingly used.5,9,21 Currently reported clearing rates range between 6% and more than 90%,21–24 which may be due to the IPL devices’ wide wavelength spectrum (a second absorbance peak of oxyhemoglobin at 555nm and a third around 900nm).5 IPL is a less studied technique, in which multiple parameters (fluence, wavelength spectrum, pulse duration, intra- and interpulse delay times) may be adjusted, conditioning an endless amount of therapeutic options.
In the present study we have been able to show the histochemical SP of IPL, even with multipulse treatments, according to the Verkruysse model25 first demonstrated with PDL treatments. Using multipulses of IPL, higher energy thresholds are reached in the vessel walls with less collateral damage.17,18,25
Single and multiple pulsesMultiples pulses produce an accumulative increase in temperature in blood vessels, leading to thermal coagulation.18 In order to maintain SP the caliber and density of the vessel walls must be taken into account.
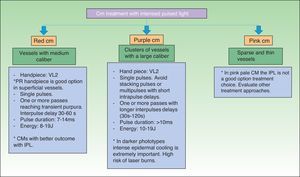
Interpulse delay times (multiple passes and stacking pulses)Multiple passes with large interpulse delays (30–60s) seem to heat the vessel walls slowly, being less aggressive than stacking pulses. This may be an advantage for patients who did not respond to a single-pulsed treatment. This treatment modality is especially useful in CM in the presence of clusters of vessels with a larger caliber, which have a longer thermal relaxation time (TRT), because it allows cooling between pulses.17 In these cases, stacking pulses may lead to overheating and consequent collateral dermal damage.
On the other hand, in patients with CM with sparse and thin vessels, short interpulse delay times or stacking pulses could be safer in fair-skinned patients and allow the necessary temperature increase to achieve thermal coagulation and consequent vessel wall destruction, without collateral damage.
Intrapulse delay timesManaging intrapulse delays is also extremely important, taking into account the color of the CM, which depends on the vessel density and caliber.15 Again, long intrapulse delays are safer in the presence of high vessel density and larger caliber. Short intrapulse delays are appropriate to treat CM lesions with low vessels¿ density and small diameter and therefore with less chromophore to absorb light energy.17
Darker phototypes and epidermal damageThe risk of epidermal injury is especially seen in patients with darker phototypes, due to considerable melanin absorption of IPL light, mainly in the basal layer.5 Besides epidermal damage, these patients also showed a poor response to treatment, with insufficient vessel heating and thermal coagulation because most of the energy was retained in the epidermis. Intense epidermal cooling is extremely important in these patients to allow light energy penetration in the dermis.26
The therapeutic approach to the CM depends on the caliber, capillary density and depth of the vessels as well as the patient's skin type (Fig. 4).
This study has many limitations, such as the heterogeneity of the selected patients (regarding the skin phototype of the patients or the color and location of the CM) and, above all, the amount of different IPL setting combinations used. However, our aim was only to start to understand the potential improvements we could achieve with the IPL device by individualizing the selection of parameters through these first histochemical observations. These observations may allow for a future comparative and controlled study in which a single or only a small number of parameters are changed when treating comparable lesions/patients in regards to the color and location of the CM and the patient's skin phototype.
In IPL treatment of CM, the goal is to take full advantage of the energy of the emitted light to selectively damage only the vessels without collateral damage. The final outcomes are conditioned by many factors. With regard to the patient, physicians should consider: the vessel size, density and depth; the quantity of red blood cells inside the vessels (i.e., the target chromophore); and the epidermal pigmentation of the patient.9,18 Regarding the IPL device and the operator, factors that influence our results are: the wavelength spectrum of the IPL device, the light energy, the pulse duration, the possibility of using single or multiple pulses with variable inter- and intrapulse delay times and choosing adequate cooling strategies.18
ConclusionsIn this study we explored the potential of an IPL device in treating CM and the huge number of possible treatment parameter combinations that can be used. IPL intra- and interpulse delay changes produce very different final results. Our findings suggest that patients with thin vessels should be treated in a more aggressive way. Perhaps with the recent introduction of next-generation intensed pulsed lights with shorter pulses can improve the treatment of this group of lesions. On the other hand, physicians treating CM in patients with darker phototypes should choose an adequate cooling strategy to avoid epidermal damage.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this research has not been conducted experiments on humans or animals.
Confidentiality of dataThe authors declare that they have followed the protocols of the workplace on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of patients and/or subjects referred to in Article consent. This document is in the possession of the corresponding author.
Conflict of interestsThe authors declare no conflict of interest.