Topical therapy is vital in the management of psoriasis. In recent years there have been multiple advances and changes in the management of psoriatic patients that justify a review and update of the use of topical therapy. Experts from the Spanish Psoriasis Working Group (GPS) of the Spanish Academy of Dermatology Venereology (AEDV) have developed a set of recommendations for the treatment of psoriasis based on the best available evidence and experts’ opinion.
MethodologyWe followed the methodology of nominal groups, with the help from a systematic review. A coordinator was designated, and a group of experts was selected based on their experience and knowledge on topical therapies for the management of psoriasis. Afterwards, the objectives and key points of the document were defined and agreed upon, and with help from a documentary specialist, a systematic review was conducted across Medline, Embase and Cochrane Library. Systematic reviews, meta-analyses, clinical trials, as well as observational studies were selected. Published clinical practice guidelines and related documents were also reviewed. With this information, the coordinator generated a series of recommendations that would be later evaluated and modified by the experts themselves. After several review processes and an external evaluation, the final document was drafted.
ResultsPractical recommendations on the use of topical therapies for the management of psoriasis are presented in line with other documents of the AEDV, including the use of topical treatment as the main therapy, their adjuvant role when using systemic therapies, treatment selection, treatment strategies, their use in special locations and severe psoriasis, and the patients adherence and preferences to the topical therapy. The document critically evaluates the safety and efficacy profile of topical therapy.
El tratamiento tópico es fundamental en el paciente con psoriasis. En los últimos años se han producido múltiples avances y cambios en el manejo de la psoriasis que hacen que se precise una revisión y actualización del uso del tratamiento tópico. Expertos del Grupo Español de Psoriasis (GPS) de la Academia Española de Dermatología y Venereología (AEDV) han generado recomendaciones sobre el tratamiento tópico de la psoriasis, basadas en la mejor evidencia disponible y la experiencia de los expertos.
MetodologíaSe siguió la metodología de grupos nominales, con ayuda de una revisión sistemática de la literatura. Se designó un coordinador y seleccionó un grupo de expertos en función de su experiencia y conocimiento en el uso del tratamiento tópico en la psoriasis. Tras ello se definieron y consensuaron los objetivos y puntos clave del documento, y con ayuda de una documentalista, se realizó una revisión sistemática de la literatura en Medline®, Embase® y Cochrane Library®. Se seleccionaron revisiones sistemáticas, metaanálisis, ensayos clínicos, así como estudios observacionales. Se revisaron otras guías de práctica clínica y documentos relacionados. Con esta información se crearon una serie de recomendaciones que fueron evaluadas y modificadas por los expertos. Tras varios procesos de revisión, y una evaluación externa se redactó el documento definitivo.
ResultadosSe presentan recomendaciones prácticas, en línea con otros documentos de la AEDV, sobre el uso de los tratamientos tópicos en la psoriasis. Se incluye el uso del tratamiento tópico como tratamiento principal y como coadyuvante del tratamiento sistémico, la selección del tipo de tratamiento tópico, las estrategias de tratamiento, su uso en localizaciones especiales y tipos de psoriasis grave, así como la adherencia y las preferencias del paciente. En el documento se valora críticamente la eficacia y seguridad de la terapia tópica.
Topical treatment is a fundamental pillar in the management of psoriasis, effective in approximately 70% of patients with mild to moderate psoriasis1 and as an adjuvant to systemic treatment in moderate-to-severe psoriasis.2,3
In 2009, the Spanish Psoriasis Working Group (GPS) of the Spanish Academy of Dermatology and Venereology (AEDV) published evidence-based recommendations on the topical treatment of psoriasis.4 Since then, substantial changes have occurred, such as the emergence of biological therapies for the treatment of moderate-to-severe psoriasis.5,6 The demonstrated superiority of these compounds in terms of efficacy, safety, and maintenance of response7 has modified therapeutic goals5,6 and contributed to the development of criteria, indications, and objective measures for assessing severity and therapeutic response.5,6
On the other hand, patient involvement in decision-making is increasingly important,5,6,8 with poor adherence to treatment even being associated with the patient's lack of acceptance of the medication.9–12
RationaleWe are in a new scenario in the treatment of psoriasis that requires a review of the role of topical treatments within the current scientific and clinical practice context. Furthermore, considering the variety of topical treatments available, it is essential to offer a treatment with proven efficacy that adapts to and is accepted by the patient. With this premise, the GPS proposes the following objectives:
- 1.
Critically evaluate the published evidence and develop a topical treatment guideline for psoriasis that serves as a reference for dermatologists.
- 2.
Create a treatment framework for patients with mild psoriasis treated in primary care that can serve as consultation material for other involved professionals.
- 3.
Generate value regarding the use of topical treatment.
The recommendations issued refer to the use of topical treatment for adult patients with mild-to-moderate psoriasis and as an adjuvant in the management of moderate-to-severe psoriasis.
MethodologyStudy design. This consensus document has been promoted by the GPS of the AEDV. The nominal group technique was used, along with a systematic literature review. The study was conducted in accordance with the principles established in the Declaration of Helsinki, on medical research in human beings in its latest version, and in full compliance with applicable regulations on Good Clinical Practice.
Selection of participants and document development. First, a coordinator was appointed, and a group of 3 experts plus members of the GPS were selected based on their experience and knowledge of psoriasis. Subsequently, the objectives, scope, users, and sections of the document were defined.
Systematic literature review (SLR). An expert documentalists designed different search strategies, including Mesh terms and free text, in the main bibliographic databases (Medline, Embase, and the Cochrane Library) up to September 2021, with a subsequent update in October 2023 (see supplementary data, annex). Systematic reviews and meta-analyses, randomized controlled trials (RCTs), real-world quality studies, clinical practice guidelines, and national and international consensus documents on the use of topical treatment in psoriasis were selected.
Based on the information collected, the coordinator generated a series of recommendations that were evaluated and modified by the experts. After external review by 2 members of the GPS who were not involved in the generation of the content and recommendations of the consensus, the final document was drafted.
ResultsGeneral considerationsDifferential characteristics of topical treatment in relation to systemic treatmentFour elements influence the therapeutic response in topical treatment: the skin, the active ingredient, the vehicle (base or excipient), and the application technique (cutaneous hydration/open or occlusive dressing). The skin's resistance to the penetration of topical treatments is variable, following an order from least to greatest: mucous membranes, scrotum, eyelids, face, chest and back, arms and thighs, forearms and legs, back of hands and feet, palms of hands and feet; and nails. The potency of the active ingredient also conditions the response, as well as the vehicle and the application technique, due to their effect on absorption and duration of action.
Due to its characteristics, topical treatments have been used for decades with different doses, vehicles, time periods, populations, and treatment strategies (alone and in combination). All of this extraordinarily limits comparability and complicates the establishment of an evidence-based usage guideline, as has been done with systemic treatments, but explains the extensive experience in using these compounds.
Finally, patient participation in psoriasis is always fundamental to achieving good therapeutic adherence, which is even more important in the case of topical treatments with cosmetic implications.
Indication of topical treatment in psoriasisTopical treatment is indicated in any patient with psoriasis, regardless of their characteristics, type, or severity. It can be used as the main treatment in mild psoriasis, and individualized cases of moderate psoriasis, or as an adjuvant to systemic therapy in patients with moderate-to-severe psoriasis (see next section). Thus, achieving the therapeutic goal will depend on topical treatment in the first case, and on systemic therapy in the second. Similarly, the evaluation of the safety and efficacy profile of topical treatment as an adjuvant will be restricted to the application area(s).
Topical treatment can also be indicated as monotherapy in patients with moderate-to-severe psoriasis who refuse systemic treatment due to fear of side effects, comorbidities, or other personal considerations. Finally, it can also be considered in patients cleared with discontinued systemic treatment or in the so-called “therapeutic vacations.”
Assessment of psoriasis severityThe criteria for assessing the severity of psoriasis in adults are described in the GPS document on the use of biological therapies.5 Based on these criteria, patients with psoriasis are classified as mild or moderate-to-severe.
Of note, topical treatments are also prescribed by primary care physicians and other health professionals who may have insufficient knowledge of disease severity assessment tools such as the Psoriasis Area Severity Index (PASI), body surface area (BSA), or the Dermatology Life Quality Index (DLQI). Therefore, the GPS includes, as an alternative, the use of the Physician's Global Assessment (PGA).
On the other hand, topical treatments are very frequently used in mild forms where some of these tools have limitations, so in certain cases, the DLQI and the patient's opinion may be more appropriate.
Finally, we should mention that, due to the extraordinary clinical heterogeneity of the disease, in daily practice we find patients who, although their severity (according to accepted criteria5) may be moderate-to-severe, give us a clinical perception closer to the moderate than severe concept, as defined by a group of expert dermatologists.13 The GPS considers that, in these cases, and on an individualized basis, topical treatment can be considered with the patient as the main treatment before indicating systemic therapy, which would correspond to them based on the accepted classification of psoriasis.5
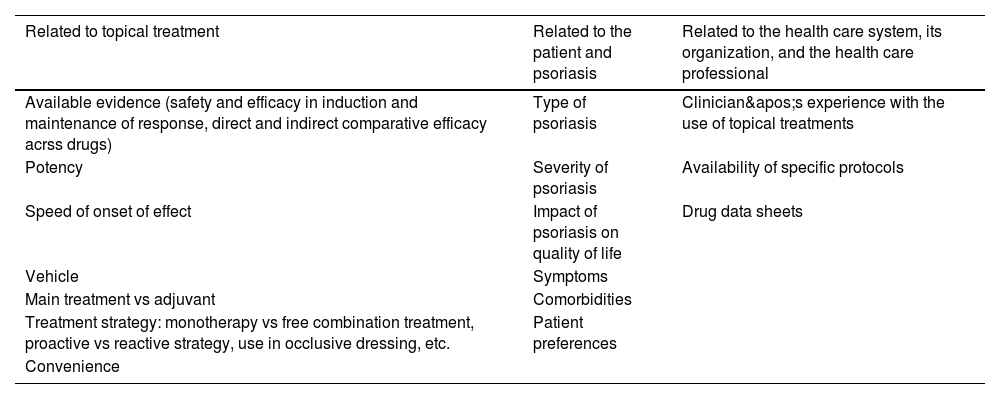
Selection of topical treatmentWhen selecting topical treatments, the criteria described for systemic treatments5 should be taken into account, with a series of considerations (Table 1). Particularly relevant factors are patient preferences and the characteristics of the vehicles, as they largely determine therapeutic adherence.
Selection criteria for topical treatment in plaque psoriasis.
| Related to topical treatment | Related to the patient and psoriasis | Related to the health care system, its organization, and the health care professional |
|---|---|---|
| Available evidence (safety and efficacy in induction and maintenance of response, direct and indirect comparative efficacy acrss drugs) | Type of psoriasis | Clinician's experience with the use of topical treatments |
| Potency | Severity of psoriasis | Availability of specific protocols |
| Speed of onset of effect | Impact of psoriasis on quality of life | Drug data sheets |
| Vehicle | Symptoms | |
| Main treatment vs adjuvant | Comorbidities | |
| Treatment strategy: monotherapy vs free combination treatment, proactive vs reactive strategy, use in occlusive dressing, etc. | Patient preferences | |
| Convenience |
Therapeutic goal is the same as that described for systemic therapies.5 Althouh the optimal one is complete skin clearance, adequate clinical criteria such as achieving a PASI 90, an absolute PASI ≤3, or a PGA 0/1 are also accepted. In addition to objective criteria, it is important to consider the patient's subjective view on their preferences and the impact of topical treatment on their quality of life.
Initial and maintenance topical treatmentUnlike systemic treatment, topical treatment does not define lines of treatment, but rather 2 different periods: an induction period and a maintenance one.
Topical treatment is prescribed continuously for a short but flexible period (generally between 4 and 12 weeks), called induction, in which the therapeutic goal is sought. From there, and to maintain the therapeutic goal, multiple long-term treatment strategies (>week 12) can be followed, such as cessation of topical treatment or changes to dose and frequency of application.
Evaluation of response to topical treatmentThe evaluation of response is conducted in the same way as with systemic therapies, that is, after the induction period and regularly during maintenance treatment. Therapeutic failure to topical treatment is a criterion for the initiation of phototherapy or systemic treatment, once explained and agreed upon with the patient.5
In some individual cases, a different approach may be considered, for example, switching to a different topical treatment before indicating systemic therapy.
Characteristics of available topical treatments for plaque psoriasis of the bodyCurrently, a wide range of topical treatments is available, with different mechanisms of action and formulated in various excipients, which can be administered in monotherapy, free or fixed combination treatment with other topical or systemic compounds, or with phototherapy, in different treatment strategies.
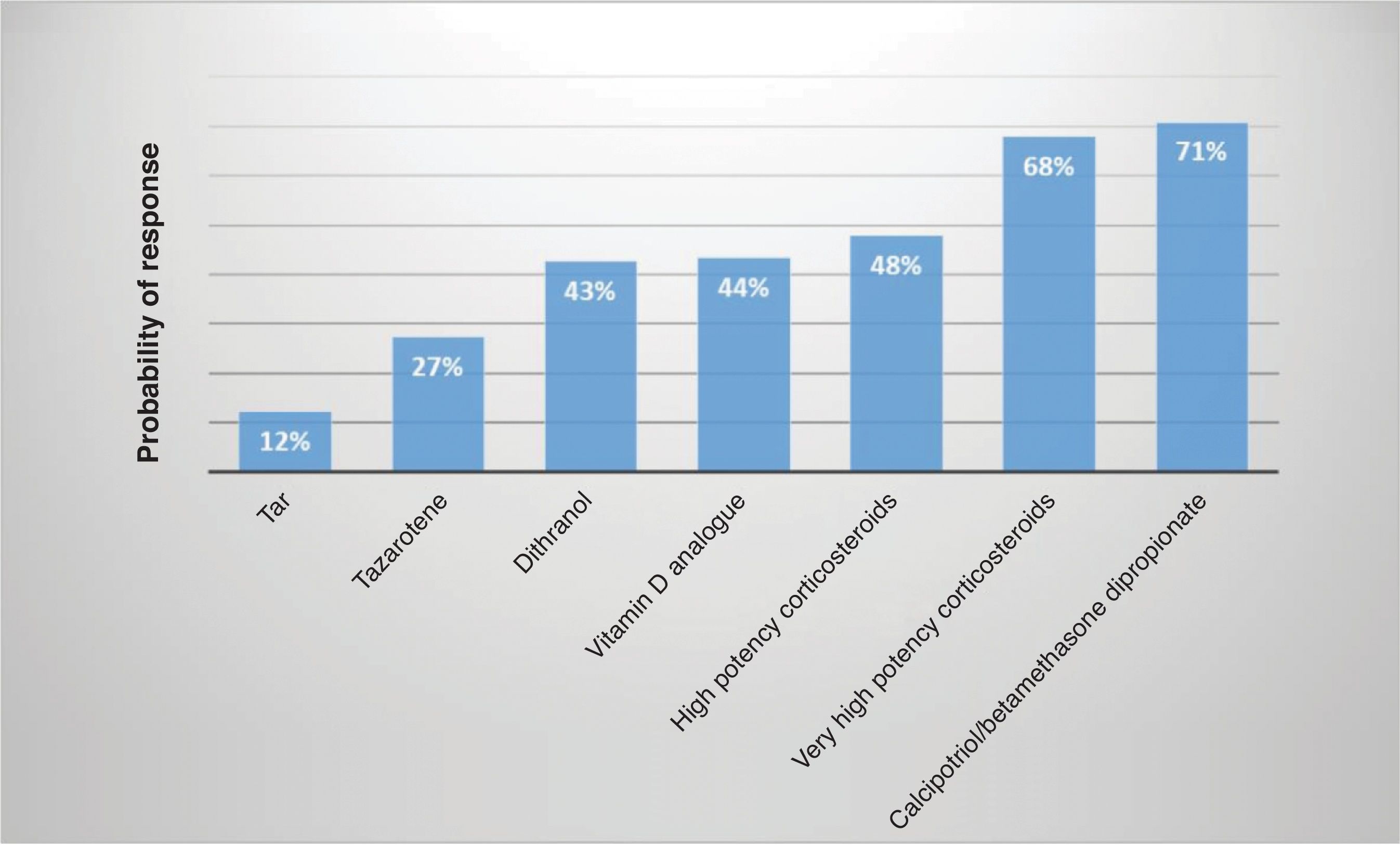
However, the available evidence on the safety and efficacy profile of these compounds is, in many cases, of low quality. Although some head-to-head studies14 are available, inter-group comparisons are inconsistent. The results of network meta-analyses (which include indirect analyses)15 and clinical experience demonstrate that there are differences across multiple topical treatments in relation to their efficacy, being very high potency corticosteroids and the fixed combination calcipotriol/betamethasone dipropionate the most effective of all (Fig. 1).
Indirect comparative efficacy of once-daily topical treatments for plaque psoriasis according to investigator assessment.
The safety profile of topical treatments is well known. They are associated with few adverse events, which are generally mild and transient. The risk of adverse events decreases with adherence to the recommendations of the technical data sheet and the prescribing physician, as well as with good patient training.
The main characteristics and usage guidelines of topical treatments are described below (Tables 2–5).
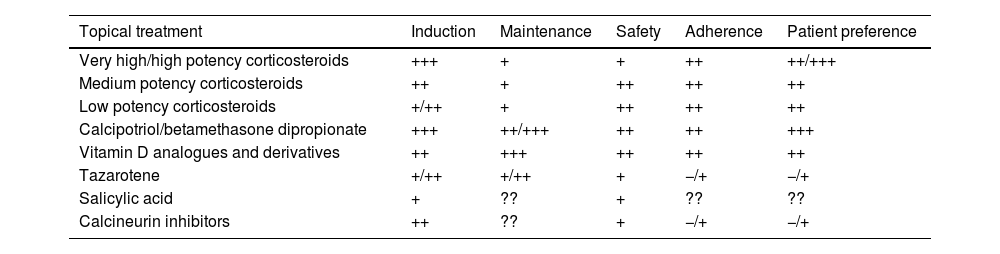
Main characteristics of topical treatments for plaque psoriasis of the body (does not include special locations or severe forms of psoriasis).
| Topical treatment | Induction | Maintenance | Safety | Adherence | Patient preference |
|---|---|---|---|---|---|
| Very high/high potency corticosteroids | +++ | + | + | ++ | ++/+++ |
| Medium potency corticosteroids | ++ | + | ++ | ++ | ++ |
| Low potency corticosteroids | +/++ | + | ++ | ++ | ++ |
| Calcipotriol/betamethasone dipropionate | +++ | ++/+++ | ++ | ++ | +++ |
| Vitamin D analogues and derivatives | ++ | +++ | ++ | ++ | ++ |
| Tazarotene | +/++ | +/++ | + | −/+ | −/+ |
| Salicylic acid | + | ?? | + | ?? | ?? |
| Calcineurin inhibitors | ++ | ?? | + | −/+ | −/+ |
Table prepared by the authors based on data found in the analyzed evidence (including indirect comparisons) and expert experience.
+++: very (effective, safe, adherent, preferred by the patient); ++: quite (effective, safe, adherent, preferred by the patient); +: somewhat (effective, safe, adherent, preferred by the patient); −/+: doubtful (effective, safe, adherent, preferred by the patient); −: not (effective, safe, adherent, preferred by the patient); ??: unknown.
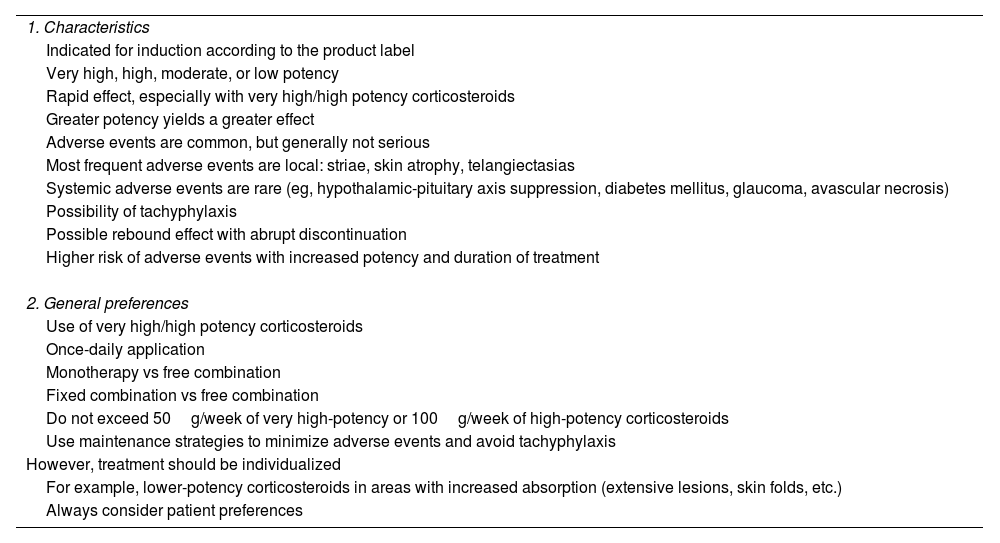
Characteristics and use of topical corticosteroids in plaque psoriasis of the body.
| 1. Characteristics |
| Indicated for induction according to the product label |
| Very high, high, moderate, or low potency |
| Rapid effect, especially with very high/high potency corticosteroids |
| Greater potency yields a greater effect |
| Adverse events are common, but generally not serious |
| Most frequent adverse events are local: striae, skin atrophy, telangiectasias |
| Systemic adverse events are rare (eg, hypothalamic-pituitary axis suppression, diabetes mellitus, glaucoma, avascular necrosis) |
| Possibility of tachyphylaxis |
| Possible rebound effect with abrupt discontinuation |
| Higher risk of adverse events with increased potency and duration of treatment |
| 2. General preferences |
| Use of very high/high potency corticosteroids |
| Once-daily application |
| Monotherapy vs free combination |
| Fixed combination vs free combination |
| Do not exceed 50g/week of very high-potency or 100g/week of high-potency corticosteroids |
| Use maintenance strategies to minimize adverse events and avoid tachyphylaxis |
| However, treatment should be individualized |
| For example, lower-potency corticosteroids in areas with increased absorption (extensive lesions, skin folds, etc.) |
| Always consider patient preferences |
g: grams.
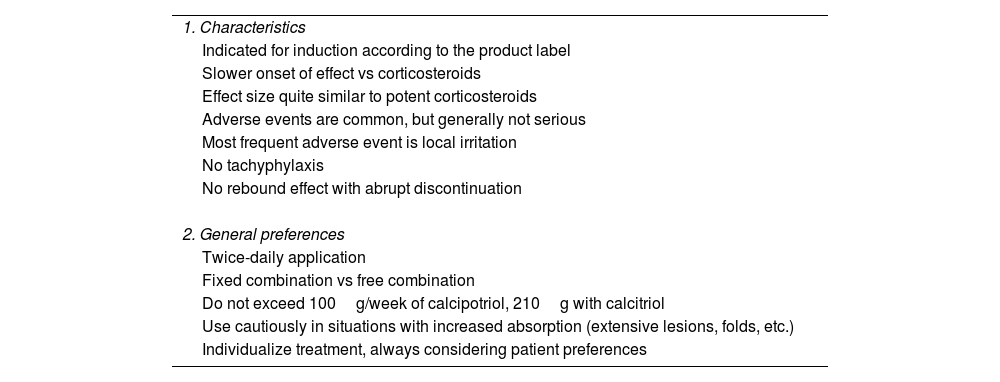
Characteristics and use of vitamin D analogues in plaque psoriasis of the body.
| 1. Characteristics |
| Indicated for induction according to the product label |
| Slower onset of effect vs corticosteroids |
| Effect size quite similar to potent corticosteroids |
| Adverse events are common, but generally not serious |
| Most frequent adverse event is local irritation |
| No tachyphylaxis |
| No rebound effect with abrupt discontinuation |
| 2. General preferences |
| Twice-daily application |
| Fixed combination vs free combination |
| Do not exceed 100g/week of calcipotriol, 210g with calcitriol |
| Use cautiously in situations with increased absorption (extensive lesions, folds, etc.) |
| Individualize treatment, always considering patient preferences |
g: grams.
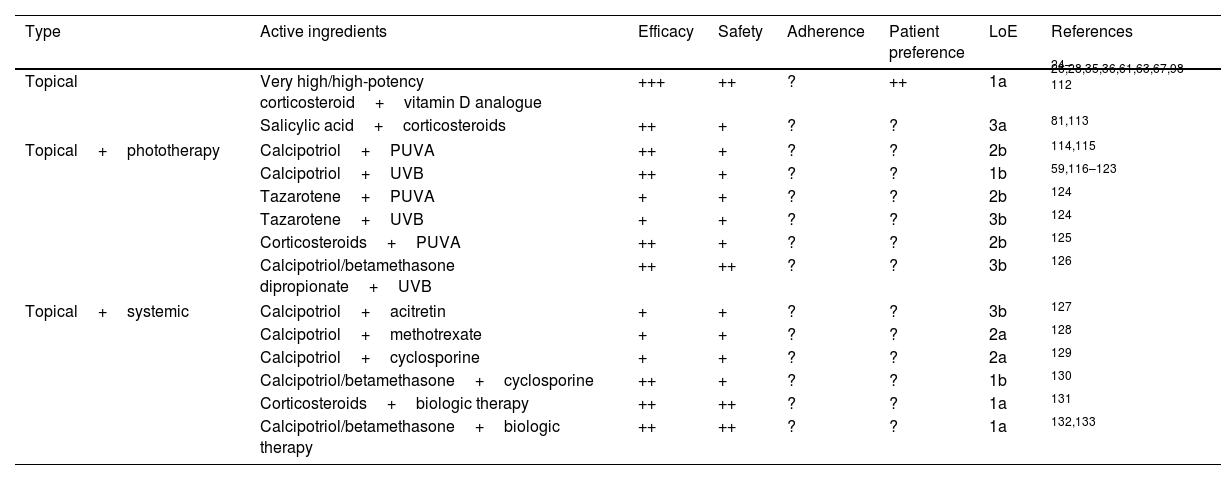
Safety and efficacy profile of topical treatments in free combination, with phototherapy, and with systemic treatments in psoriasis.
| Type | Active ingredients | Efficacy | Safety | Adherence | Patient preference | LoE | References |
|---|---|---|---|---|---|---|---|
| Topical | Very high/high-potency corticosteroid+vitamin D analogue | +++ | ++ | ? | ++ | 1a | 24–26,28,35,36,61,63,67,98–112 |
| Salicylic acid+corticosteroids | ++ | + | ? | ? | 3a | 81,113 | |
| Topical+phototherapy | Calcipotriol+PUVA | ++ | + | ? | ? | 2b | 114,115 |
| Calcipotriol+UVB | ++ | + | ? | ? | 1b | 59,116–123 | |
| Tazarotene+PUVA | + | + | ? | ? | 2b | 124 | |
| Tazarotene+UVB | + | + | ? | ? | 3b | 124 | |
| Corticosteroids+PUVA | ++ | + | ? | ? | 2b | 125 | |
| Calcipotriol/betamethasone dipropionate+UVB | ++ | ++ | ? | ? | 3b | 126 | |
| Topical+systemic | Calcipotriol+acitretin | + | + | ? | ? | 3b | 127 |
| Calcipotriol+methotrexate | + | + | ? | ? | 2a | 128 | |
| Calcipotriol+cyclosporine | + | + | ? | ? | 2a | 129 | |
| Calcipotriol/betamethasone+cyclosporine | ++ | + | ? | ? | 1b | 130 | |
| Corticosteroids+biologic therapy | ++ | ++ | ? | ? | 1a | 131 | |
| Calcipotriol/betamethasone+biologic therapy | ++ | ++ | ? | ? | 1a | 132,133 | |
LoE: level of evidence; PUVA: psoralen+UVA radiation; UVB: ultraviolet B radiation; +++: very (effective, safe, adherent, patient-preferred); ++: quite (effective, safe, etc.); +: somewhat; ±: questionable; −: not effective/safe/etc.; ?: unknown.
Topical corticosteroids are classified into very high, high, moderate, and low potency, according to the size of effect,16 and are marketed in multiple vehicles. Their technical data sheet indicates them for induction treatment, but not for maintenance. For maintenance, recommendations in the technical data sheets vary from one drug to another. They are generally focused on the use of lower doses or the shortest possible time and always under medical evaluation.
Evidence from RCTs of heterogeneous quality, and from SLRs and meta-analyses, shows that they are very effective therapies in induction, both in monotherapy and in free or fixed combination with other topical or systemic therapies in the management of plaque psoriasis of the body regardless of its severity, with a good safety profile (Tables 2 and 3).3,14,15,17,18
The effect appears rapidly and reaches its maximum at 2–4 weeks.3,14,15,17,18 The greater the potency of the corticosteroid, the greater the size and duration of the effect, but the greater the risk of adverse events too.14,18 Two weeks into therapy, the response rate in objective variables is 58–92% for very high/high potency corticosteroids14,18–21; 70–83% for moderate potency22,23; and 60–70% for low potency.3,23 They are effective with 124–26 and 2 applications per day,19,27–32 and although it is somewhat superior with the latter regimen, as indicated in most technical data sheets, the difference is not clinically relevant, especially in the case of very high/high potency corticosteroids.4,15
Regarding maintenance treatment, although not indicated in the technical data sheet, the beneficial effect of topical corticosteroids can be extended over time, especially with very high/high potency ones.33–36 However, their prolonged use is associated with a higher risk of adverse events and the appearance of tachyphylaxis.2,4
Regarding safety, adverse events are generally mild, especially if the technical data sheet's risk management instructions are followed.14 The most common local presentations are skin atrophy, striae, folliculitis, telangiectasias, and purpura.37,38 Abrupt cessation of application could have a rebound effect, although this phenomenon is not well characterized. Adverse events due to systemic absorption are exceptional.37–42 The risk of adverse events increases: in continuous treatments, with a larger amount of treated body surface area (BSA ≥20%), in occlusive applications, in special populations such as children and the elderly, in the presence of severe comorbidity, such as renal or hepatic insufficiency; and in certain locations such as the face and folds.4 In all these circumstances, close monitoring of the patient is recommended.
Fixed combination of calcipotriol/betamethasone dipropionateThe fixed combination of calcipotriol/betamethasone dipropionate has been approved for use in induction and is available in cream, ointment, gel, and foam. In addition, the foam has an indication in the technical data sheet as a proactive maintenance strategy.43,44 Its safety and efficacy profile has been evaluated in patients with plaque psoriasis in multiple quality RCTs, both in induction and maintenance, regardless of the patient's age and severity.45–48 It has a rapid effect with a mean PASI reduction of 65-74% at 4 weeks and superior efficacy to monotherapy. Application twice a day does not achieve greater efficacy than once a day.35
The results of an RCT comparing the fixed combination in foam vs its vehicle showed a therapeutic success rate of 53.3% vs 4.8% (p<0.001), with a final mPASI score of 2 vs 5.5 (p<0.001) 4 weeks into therapy. A total of 83.5% of patients reported a rapid and significant decrease in pruritus45 and improved quality of life.46 The foam is superior to the gel,47 with a therapeutic success rate (PGA 0/1) of 44.1% vs 34.3%, respectively 12 weeks into therapy.46 Sub-analysis of patients with moderate-to-severe psoriasis showed a higher frequency of mPASI75 response with the foam vs the gel (57.1% vs 35.4%; p=0.006) 12 weeks into therapy. The therapeutic success rate was numerically higher with the foam from week 4 to 12.47
Aggregated data from 2 RCTs49,50 have shown significantly superior differences of the cream vs the gel in different outcome variables such as therapeutic success rate, mPASI (−64.6% vs −56.4%; p<0.001), mPASI75, and improvement in DLQI (6.5 vs 5.6; p<0.001).49,50
In addition, the foam has high acceptability, satisfaction, and convenience for patients,46 being one of the formulations that has shown the highest degree of satisfaction.46,47,49–56
Regarding the safety of the fixed combination of calcipotriol/betamethasone dipropionate, adverse events are rare and generally mild, being irritation and pruritus35 the most common.
Vitamin D analoguesCalcitriol, calcipotriol, and tacalcitol are marketed in Spain with different vehicles. Their technical data sheet indicates them as induction treatment, but not as maintenance treatment.
The safety and efficacy profile of vitamin D analogues in the treatment of plaque psoriasis have been studied in various SLRs, meta-analyses, and RCTs (Tables 2 and 4).18,57,58
These compounds have shown efficacy after 4–8 weeks of treatment, especially in mild or moderate psoriasis, and severe psoriasis, with significant improvement in symptoms and signs, PASI, total severity score (TSS), patient assessment of global improvement (PAGI), and patient satisfaction.18,57,58 Clinical response is somewhat slower vs topical corticosteroids, but the size of the effect appears similar to that of potent corticosteroids.18 Although more quality comparative studies are needed, calcipotriol may be more effective than calcitriol.58–60
Administration once24,61,64 and twice a day28,30,62–66 has proven effective. On the other hand, the twice-daily formulation has been shown to be superior in an RCT,67 as shown in the technical data sheet.
Regarding maintenance treatment, although they do not have an indication, these compounds have shown a greater effect vs topical corticosteroids. In an RCT of calcitriol vs betamethasone applied twice daily, a similar mean reduction in PASI was observed in both groups after 6 weeks into therapy, although the remission maintenance rate was significantly higher with calcitriol (48% vs 25%; p<0.01).30 The results of an SLR and real-world practice data have demonstrated the safety and efficacy profile of topical calcipotriol for up to 52 weeks in a reactive strategy or up to 20 weeks in a proactive strategy.57 Its intermittent use along with topical corticosteroids has also proven effective in maintenance.34–36
Topical vitamin D analogues are safe drugs.14 The most common adverse event is lesional and perilesional irritation, described in up to 35% of cases. It is more frequent with calcipotriol than with calcitriol4 and can be reduced by decreasing the frequency of application or associating topical corticosteroids. Adverse events tend to remit during maintenance treatment,57,64,68 and systemic ones are very rare.14
The risk of adverse events increases with a larger amount of treated body surface area (BSA ≥30%), in special populations (eg, the elderly and children), in the presence of severe comorbidity (eg, renal insufficiency or disorders of calcium metabolism), and in pustular and erythrodermic psoriasis. In these contexts, closer monitoring of the patient and dose titration are recommended if needed. Regarding pregnancy, vitamin D analogues are ill-advised during pregnancy unless clearly necessary.
TazaroteneTazarotene is a retinoid that can be used in monotherapy or in combination with topical corticosteroids to increase its efficacy profile and improve tolerance.
Studies on topical tazarotene have been developed primarily in mild or moderate psoriasis and in combination treatment in moderate-to-severe psoriasis,69–71 with different vehicles, at concentrations of 0.05 and 1%, once daily, and in some isolated studies, twice daily72 (Table 2).
The use of tazarotene for 6–12 weeks (in nail psoriasis up to 24 weeks73) has demonstrated efficacy on the symptoms and signs of psoriasis, as well as on BSA, PGA, patient satisfaction, or acceptability. The onset of the effect is rapid, although somewhat slower than that of topical corticosteroids.74 In the absence of robust comparative studies, its efficacy seems to be lower than other topical therapies (Fig. 1).75–77 Although consistent data on maintenance treatment are lacking, it could be an effective drug.69
The maximum recommended dose is 100g per week, in one application per day, and in combination with corticosteroids.
Tazarotene is a safe compound with minimal systemic absorption. The most frequent adverse events are local lesional and perilesional irritation, which increases with higher concentrations of the compound.78 Some strategies to reduce the risk of adverse events are the use of cream formulation, the use of a lower concentration, intermittent application with short-contact (30 to 60min) emollients, or combination with topical corticosteroids.2,78,79 Mutagenic or cardiogenic effects have not been demonstrated.80 However, as it is a retinoid, its use is contraindicated in pregnancy.
SalicylatesThe safety and efficacy profile of topical salicylates at different concentrations has been evaluated in mild or moderate psoriasis and in moderate-to-severe psoriasis, especially in combination with topical corticosteroids (Table 2).81
Treatment with salicylic acid (from 2 to 12 weeks) has demonstrated effective (improvement of symptoms and signs, PASI, PGA, and patient acceptance) in multiple RCTs, both in monotherapy and in combination with other topical therapies.81–86 In this regard, it has been observed that the efficacy of the combination of salicylates and topical corticosteroids is superior to that of topical corticosteroids in monotherapy,81,86,87 probably because salicylic acid promotes the penetration of the corticosteroid into the skin.
Efficacy has been demonstrated with the administration of salicylates once85,88,89 and twice a day,81,82,86,87 as well as with different vehicles. Comparative data (outside of combinations) with other topical treatments are scarce, and information for maintenance treatment is not available.
The most frequent adverse event is local irritation (burning sensation and pruritus), and no serious systemic adverse events have been described. Systemic absorption is greater in patients with renal or hepatic disease and when applied to a larger body surface area (BSA >20%); therefore, it should be used with caution in these patient groups.81,84,87,90 Finally, topical salicylic acid should not be used before UVB phototherapy because it can reduce its efficacy.91
Calcineurin inhibitorsTopical tacrolimus (TAS) and pimecrolimus do not hold an indication in the technical data sheet for the treatment of plaque psoriasis.
Their molecular size and limited skin penetration capacity limit their efficacy profile in psoriasis of the trunk and extremities, so they are generally used in areas of thinner skin (face and folds) and as corticosteroid-sparing agents.
In special locations, they have demonstrated efficacy at different concentrations (TAS 0.1% and 0.03%, pimecrolimus 1%) for 4–12 weeks, in applications once92,93 and twice a day,82,93–96 with the use of different vehicles. The onset of the effect is rapid. However, outside of special locations, no significant differences have been observed between topical TAS and placebo at 6 weeks of treatment.93
Robust data on their efficacy in maintenance treatment are not available.
Safety data come primarily from studies of patients with atopic dermatitis.97 The most frequent adverse events are burning and pruritus, which may improve with continuous use and avoiding application to moist skin.
Selection and strategies for topical treatmentWhen selecting topical therapy, the following should be decided: (1) active ingredient to use; (2) induction treatment (first 2–4 weeks): monotherapy or free or fixed combination therapy, application technique (open or occlusive dressing), number of daily applications, and type of vehicle; and (3) strategy during maintenance (proactive or reactive).
The GPS recommends following the criteria defined previously and always taking into account patient preferences, as their involvement in shared decision-making is essential for achieving good adherence.
Based on the published evidence (risk–benefit profile and patient acceptance), and on the absence of contraindications, the GPS recommends prioritizing the use of a very high/high potency topical corticosteroid and the fixed combination calcipotriol/betamethasone dipropionate, except in special locations and types of psoriasis (see corresponding sections). This decision should be individualized for each patient.
The “extra” benefit of applying twice a day is not clinically relevant for most treatments and may negatively affect adherence or safety, so the once-daily regimen is preferred.
Below, we describe the most relevant aspects (see also previous tables).
Free combined topical treatment vs monotherapyIn monotherapy, a single active ingredient is used; in fixed combination, 2 active ingredients in a single application; and in free combination, 2 or more active ingredients administered separately (e.g., alternately).
- –
Due to the adherence problems associated with free combination therapy, the GPS prefers the use of monotherapy or fixed combination therapy, especially in mild forms of psoriasis.
- –
In more severe types of psoriasis, the use of topical treatment seeking the greatest possible efficacy will be evaluated (e.g., use of very high potency corticosteroids, fixed combination calcipotriol/betamethasone dipropionate, etc.).
- –
In more severe cases, and always in agreement with the patient, the use of free combination therapy will be evaluated.
- –
In patients with therapeutic failure after induction topical treatment in monotherapy or fixed combination, in whom systemic therapy is not yet considered, free combination with another topical treatment will preferably be evaluated. However, other options such as twice-daily monotherapy (if used only once), increasing the potency of the corticosteroid (if a lower potency one was used), or switching to a different monotherapy treatment with a different mechanism of action will always be individualized.
Although free combinations have been widely used in clinical practice, they have currently been replaced by fixed combinations, especially in cases of combination of corticosteroids and vitamin D analogues (Table 5).
The free combination of topical corticosteroids and vitamin D analogues has been used in psoriatic patients of different severity and location (Table 5). Corticosteroids of different potencies (generally very high/high) such as betamethasone dipropionate have been combined with calcipotriol as a vitamin D analogue. The results of quality SLRs and individual RCTs14,18,43,47,51–57,134–136 have demonstrated their efficacy, both in induction and maintenance, with a superior effect of the free combination of very high potency corticosteroids with vitamin D analogues vs monotherapy.67 The effect is established rapidly, even within the first 2 weeks in most severe cases, and is maintained long-term.57,137,138 Regarding the safety profile, adverse events are not very frequent, most are mild to moderate,57,139 with lesional and perilesional skin irritation being the most common.
The efficacy profile of the free combination of topical corticosteroids and salicylates (Table 5) has been analyzed in multiple studies, including a SLR,140 although most are of low quality.81,86,87,90,141 Its use is based on the increased penetration of topical corticosteroids in the presence of salicylic acid.142 The use of this combination for 3–15 weeks has demonstrated efficacy (symptoms and signs, PGA, PASI, or quality of life) in psoriatic patients of different severity and location, with a rapid onset of effect and generally superior to that of its components in monotherapy.140,143 Data on maintenance are not available except in nail psoriasis (see corresponding chapter). Safety data are those expected with the use of these topical therapies.
On the other hand, a 12-week prospective observational study has recently been published confirming that the use of the fixed combination of calcipotriol/betamethasone dipropionate foam and phototherapy (UVB) was significantly superior to phototherapy in monotherapy (skin clearance, PASI, or itching) and managed to reduce the number and cumulative dose of phototherapy126 (see Table 5).
The quality of evidence on the safety and efficacy profile of other free combinations of topical therapies is low and, furthermore, they are not usually used in the routine clinical practice. They include the free combination of topical corticosteroids with tazarotene,69,74,77,144–148 and calcineurin inhibitors,149 vitamin D analogues with tazarotene,150 and calcineurin inhibitors,151,152 and salicylates with calcineurin inhibitors82 (Table 5).
Proactive vs reactive topical treatment in maintenance- –
The goal of maintenance treatment is to preserve the therapeutic goal until the (ideally) suspension of topical therapy.
- –
Maintenance treatment should be flexible (intensification and de-intensification) and adapted to each patient's characteristics, psoriasis, and treatments.
- –
The use of a proactive or reactive strategy should be decided individually and in agreement with the patient.
- –
If the patient improves with induction treatment but does not achieve the therapeutic goal, it will be extended for another 4–8 weeks, and in cases without response, systemic treatment or phototherapy will be considered.
- –
If the therapeutic goal is achieved with induction treatment with complete skin clearance, different maintenance strategies will be evaluated, such as topical treatment discontinuation, gradual reduction (e.g., every 2 days, then twice a week), switching to a lower potency corticosteroid or a vitamin D analogue, with subsequent suspension.
- –
If the therapeutic goal is achieved with induction treatment, but not complete skin clearance, a more conservative maintenance strategy will be evaluated.
- –
If therapeutic goal is lost with maintenance treatment, its intensification or return to the original topical treatment for 4–8 weeks with subsequent evaluation of maintenance will be considered.
Many psoriatic patients achieve the therapeutic goal with short courses of treatment (8–12 weeks and, in some cases, even in 4 weeks), although others will require more time. In any case, in long-term treatment, the possibility of flares or phases of worsening must always be considered. A SLR with a quality meta-analysis has shown that, after the suspension of topical treatment, the risk of relapse is 20%–80% at 4–8 weeks, and can increase up to 88% at 6 months.15 Therefore, it is important to maintain the therapeutic goal, once achieved, to prevent the appearance of new flares.
In the selection of maintenance treatment, the risk/benefit profile of the topical treatment, patient preferences, and adherence must be taken into account. In addition, a flexible therapy should be used that allows for intensification or de-intensification and helps to minimize the need for systemic treatment.153,154
Maintenance strategies are categorized as proactive or reactive. The proactive, preventive, or intermittent strategy consists of maintaining treatment of previously affected areas 1 or 2 times per week to prevent flares. The reactive strategy is based on the suspension or progressive de-intensification of topical treatment until its suspension (e.g., dose or frequency reduction, use of a lower potency corticosteroid or a vitamin D analog in monotherapy), with subsequent use (on demand) in case of flare or worsening.
Although there is no evidence on proactive strategies for all topical treatments, experts consider that they can be used with any of them.2 Similarly, there is no robust evidence on the superiority of one specific strategy over another.
The results of various RCTs have shown that the beneficial effect of topical corticosteroids during induction can be prolonged with proactive (intermittent) or reactive (on demand) strategies, especially with very high/high potency corticosteroids, with or without other associated topical treatments.33,155,156
Data from quality studies indicate that topical calcipotriol monotherapy is safe and effective for up to 52 weeks in a reactive strategy, or up to 20 weeks in a proactive regimen.57 In >1-year reactive treatments, the safety and efficacy profile of the fixed combination of calcipotriol/betamethasone dipropionate is better than that of calcipotriol monotherapy.57
Of note, an RCT in which, after 8 weeks of induction treatment with the fixed combination of calcipotriol/betamethasone dipropionate gel once daily, responding patients (total or almost total clearance) were randomized to 8 weeks of maintenance treatment with one of the following strategies: reactive on demand once daily; proactive (fixed dose) once daily; proactive (intermittent) twice weekly.157 Response rate was 69.9% with the on-demand or reactive strategy and 67.5% with the fixed-dose proactive strategy, significantly higher than the 31.4% with the intermittent proactive strategy (p<0.050 in both cases). In this RCT, there were no differences in safety between the different strategies.157
Recently, the results of a quality RCT designed to evaluate 52-week maintenance with a fixed combination of calcipotriol/betamethasone dipropionate foam, following 2 strategies (proactive and reactive), have been published&.43 In a previous open-label phase, patients with a response (total or almost total clearance) to this therapy once daily for 4 weeks44 were selected and randomized to a proactive maintenance strategy (intermittent, combination twice weekly), or a reactive strategy (vehicle use twice weekly and combination once daily if a flare occurred). Patients in the proactive group had a longer duration of remission (41 days more) than those in the reactive group (p<0.001) with a lower number of flares (3.1 vs 4.8, respectively).43
The fixed combination of calcipotriol/betamethasone dipropionate has demonstrated efficacy in scalp psoriasis, in both proactive and reactive strategies, although with better results for the former.158
An intervention study of a program to improve adherence and optimize topical treatment in psoriasis (topical treatment optimization program [TTOP]) involved 1800 patients with mild-to-moderate psoriasis who received the fixed combination of calcipotriol/betamethasone dipropionate gel once daily for 8 weeks, followed by another 56 weeks with a reactive strategy (on demand). Patients were randomized to a specific program to improve adherence or to usual follow-up. Eight weeks into therapy, the response rate (total or almost total clearance according to PGA) was significantly higher in the TTOP group (36.3% vs 31.3%; p=0.026). These differences were maintained from week 8 to 64, with no differences observed in other outcome variables such as BSA, patient assessment, or DLQI.159
Adjuvant topical treatment to systemic treatment and phototherapy- –
The use of topical treatment as an adjuvant is recommended in residual lesions that do not respond completely to systemic treatment or phototherapy, as a systemic treatment-sparing strategy in patients with safety concerns, or for cost optimization.
- –
The selection criteria and treatment strategy are the same as those previously discussed for other scenarios.
The use of topical treatments as an adjuvant to systemic drugs – both biological and non-biological – as well as to phototherapy, can improve efficacy (e.g., in isolated lesions with incomplete response), and also improve safety and reduce costs, since acting as systemic treatment-sparing agents lowers the risk of adverse events and treatment cost (Table 5).
Various studies have demonstrated the efficacy of calcipotriol combined with psoralens and ultraviolet A radiation (PUVA) or ultraviolet B radiation (UVB).59,116–123,160,161 It has been published that topical tazarotene at 0.1% may enhance the efficacy of phototherapy and reduce the amount of radiation needed to achieve clinical improvement.124
On the other hand, combining topical corticosteroids with PUVA has also proven effective in patients with palmoplantar psoriasis.125
In a RCT that included 135 patients with severe psoriasis, the combination of acitretin with topical calcipotriol ointment twice daily resulted in a significantly higher clearance rate vs acitretin monotherapy (67% vs 41%; p=0.006), with no safety differences.162
Regarding classical systemic treatments, one RCT showed that, in patients on standard weekly doses of methotrexate (MTX), the adjuvant use of topical calcipotriol ointment twice daily reduced the required MTX dose to maintain response (from 9.9 to 6.5mg per week; p=0.002), and increased the time to flare-up.128
The combination of calcipotriol with low-dose cyclosporine has been used in various studies. Results of an RCT involving 69 patients with severe psoriasis showed that calcipotriol ointment as an adjuvant to cyclosporine at 2mg/kg/day achieved a higher total clearance rate or PASI 90 than cyclosporine monotherapy (50% vs 11.8%; p=0.002), with no safety differences.129 In another open-label RCT with patients with moderate-to-severe psoriasis, combining cyclosporine 2mg/kg/day with a fixed-dose combination of calcipotriol/betamethasone dipropionate ointment once daily achieved a significantly higher PASI 75 after 8 weeks than low-dose cyclosporine monotherapy (87% vs 37%; p=0.0001).130
Regarding the co-adjuvant use of topical treatments with biologic therapies, data are available for anti-TNF agents131,163–166 and ixekizumab.167 An RCT of 600 psoriatic patients analyzed etanercept (ETN) with or without on-demand topical clobetasol foam as adjuvant therapy. At 12 weeks, the percentage of patients achieving PASI 90 was significantly higher in the combination group than with ETN monotherapy (29.7% vs 19.4%; p=0.009).131 However, no significant differences in efficacy or safety were observed at 24 weeks.131 These results were very similar to those of another open-label RCT using ETN with various on-demand topical therapies (hydrocortisone, betamethasone valerate, betamethasone dipropionate, clobetasol, calcitriol, or calcipotriol/betamethasone dipropionate).163,168
Results obtained with adalimumab (ADA) are very similar to those of ETN. In one RCT, using the fixed combination of calcipotriol/betamethasone once daily as adjuvant to ADA showed significantly higher efficacy than ADA monotherapy, but only within the first 4 weeks of treatment.132 A subgroup analysis of this study confirmed the same results in patients with scalp, nail, or palmoplantar psoriasis.133
An open-label study of patients with suboptimal response to ETN or ADA analyzed the adjuvant use of calcipotriol/betamethasone dipropionate foam.165 This was associated with notable improvements in BSA and PGA scores and reduced costs by avoiding switches to more expensive biologics.165
On the other hand, in 25 patients with residual psoriasis after 24 weeks of ixekizumab treatment, the adjuvant use of fixed-dose calcipotriol/betamethasone dipropionate foam once daily for 4 weeks, and every other day for 8–12 additional weeks, resulted in significant improvements in BSA and PGA parameters.167
Open dressing or occlusive treatment- –
Occlusive topical treatment can be considered on a case-by-case basis for well-defined, localized, and recalcitrant plaques, with extreme caution and for the shortest possible duration due to the risk of adverse events.
Occlusion enhances drug penetration and increases efficacy and speed of action, but also raises the frequency and severity of adverse events due to increased systemic absorption. In general, it is recommended that occlusion not exceed 12h and be used preferably at night. Occlusion can be performed with hydrocolloid patches, self-adhesive dressings, plastic films, transparent membranes, etc., and patient instruction is very important.
This technique has been used in recalcitrant, localized psoriasis lesions anywhere on the body,169–171 including the scalp,172 nails,73,173 palms and soles,89,174,175 and in localized pustular psoriasis.176
Topical corticosteroids (generally of very high/high potency) are most commonly used under occlusion,169,177 over variable durations (from 11 days to 5 weeks).169,177
In an RCT comparing once-daily occlusive treatment with betamethasone valerate vs twice-daily ointment over 3–5 weeks in 231 patients with mild-to-moderate psoriasis, occlusion led to significantly better skin clearance, PGA scores, and patient satisfaction at weeks 3 and 5,169 with no differences in flare-up rates three months after treatment cessation (13.6% in the occlusive group vs 10.4% in the ointment group). In another non-inferiority RCT in mild-to-moderate psoriasis, betamethasone valerate under occlusion for 4 weeks was not inferior in efficacy (PGA, quality of life) or safety vs the fixed-dose calcipotriol/betamethasone dipropionate ointment.170
An open-label RCT in 61 patients with mild-to-moderate psoriasis compared hydrocolloid patch occlusion with once-weekly clobetasol lotion vs twice-daily clobetasol ointment for 6 weeks.171 Occlusive therapy resulted in faster effects and 100% clearance vs 69% with topical application (p=0.005). Time to flare-up after treatment cessation was shorter with occlusion (25 vs 40 days), though not statistically significant. Adverse events were infrequent and mild, with skin irritation being the most common.171
Isolated reports have shown good results with occlusive use of vitamin D analogs, calcineurin inhibitors, tazarotene, steroid-free combinations, corticosteroids with TAS 0.1%, as well as with the fixed-dose calcipotriol/betamethasone dipropionate ointment.73,149,172–174,178
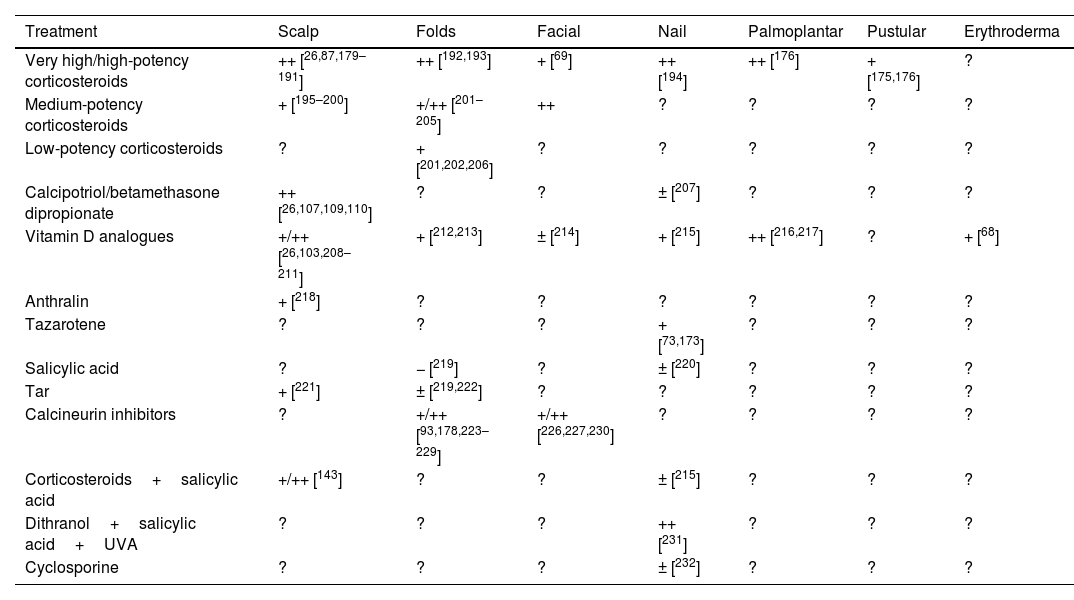
Utility and suitability of topical treatment in special locations and severe psoriasis typesA summary of the evidence and the GPS position on the use of topical treatment in special locations and severe psoriasis types is shown in Tables 6 and 7. This is described in more detail below.
Efficacy of topical treatments in special locations and severe forms of psoriasis.
| Treatment | Scalp | Folds | Facial | Nail | Palmoplantar | Pustular | Erythroderma |
|---|---|---|---|---|---|---|---|
| Very high/high-potency corticosteroids | ++ [26,87,179–191] | ++ [192,193] | + [69] | ++ [194] | ++ [176] | + [175,176] | ? |
| Medium-potency corticosteroids | + [195–200] | +/++ [201–205] | ++ | ? | ? | ? | ? |
| Low-potency corticosteroids | ? | + [201,202,206] | ? | ? | ? | ? | ? |
| Calcipotriol/betamethasone dipropionate | ++ [26,107,109,110] | ? | ? | ± [207] | ? | ? | ? |
| Vitamin D analogues | +/++ [26,103,208–211] | + [212,213] | ± [214] | + [215] | ++ [216,217] | ? | + [68] |
| Anthralin | + [218] | ? | ? | ? | ? | ? | ? |
| Tazarotene | ? | ? | ? | + [73,173] | ? | ? | ? |
| Salicylic acid | ? | − [219] | ? | ± [220] | ? | ? | ? |
| Tar | + [221] | ± [219,222] | ? | ? | ? | ? | ? |
| Calcineurin inhibitors | ? | +/++ [93,178,223–229] | +/++ [226,227,230] | ? | ? | ? | ? |
| Corticosteroids+salicylic acid | +/++ [143] | ? | ? | ± [215] | ? | ? | ? |
| Dithranol+salicylic acid+UVA | ? | ? | ? | ++ [231] | ? | ? | ? |
| Cyclosporine | ? | ? | ? | ± [232] | ? | ? | ? |
UVA: ultraviolet A radiation; +++: very effective; ++: quite effective; +: somewhat effective; ±: questionable efficacy; −: not effective; ?: unknown efficacy.
Selection of treatment (recommended and alternative) for special locations and severe forms of psoriasis.
| Location or type of psoriasis | Recommended topical treatment and alternative (after “/”) | Expected time to response |
|---|---|---|
| Scalp | Very high/high potency corticosteroids calcipotriol/betamethasone dipropionateCombination of corticosteroids and salicylic acidVitamin D analogs | 4 weeks |
| Skin folds | Medium/low potency corticosteroidsCalcineurin inhibitors | 2 weeks |
| Face | Medium/low potency corticosteroidsCalcineurin inhibitors | 2 weeks |
| Nail | Very high/high potency corticosteroids Calcipotriol/betamethasone dipropionateCalcineurin inhibitors or tazarotene | 4 weeks |
| Palmoplantar | Very high/high potency corticosteroidsCalcipotriol/betamethasone dipropionateVitamin D analogs | 4 weeks |
| Erythrodermic | Very high/high potency corticosteroids | 4 weeks |
| Localized pustular | Very high/high potency corticosteroids | 4 weeks |
- –
Scalp psoriasis can be difficult to treat. The GPS recommends the use of very high/high potency topical corticosteroids or the fixed combination of calcipotriol/betamethasone dipropionate. Other alternatives include corticosteroid-free combinations with salicylic acid or vitamin D analogues.
Efficacy studies on topical treatments for scalp psoriasis, including combination therapies, have produced variable results3,15,17 (Table 6).
Regarding topical corticosteroids, we highlight a 2017 Cochrane systematic review that analyzed 59 RCTs including 11,561 patients with mainly mild-to-moderate scalp psoriasis. This review found that corticosteroids and their combination, either free or fixed, with vitamin D analogues were significantly superior to vitamin D analogues alone in terms of global physician and patient assessments, quality of life, and treatment withdrawal due to adverse events.17 Other studies have shown that high-potency corticosteroids demonstrate similar efficacy to very high-potency corticosteroids,95,181,187,189,197,200,233 though with a lower level of evidence.
In general, published studies report a treatment duration with topical corticosteroids of 4–8 weeks, although data exist for up to 12 weeks of treatment.17,158 No significant differences have been observed between once-daily and twice-daily applications,180 and several studies have found that foam formulations are superior to solutions, but not to lotions.190,196
Similarly, the efficacy profile of the fixed combination of calcipotriol/betamethasone dipropionate has proven superior to monotherapy with topical corticosteroids, although the size of the effect is small.26,107,109,110
On the other hand, one RCT compared 2 treatment strategies using the fixed combination of calcipotriol/betamethasone dipropionate in gel: proactive (twice weekly) vs reactive (as needed). The authors found both strategies to be effective, but twice-weekly use was significantly more effective than use on demand.158
The most common adverse effects with the use of corticosteroids and vitamin D analogues (in monotherapy or combination) in patients with scalp psoriasis are local irritation, pain, and folliculitis. Systemic adverse events are very rare and likely unrelated to the treatments.17
As noted, there are few long-term data on the use of corticosteroids or vitamin D analogues in scalp psoriasis.111 The results of a 52-week RCT showed that the fixed combination of betamethasone dipropionate/calcipotriol gel was significantly superior to calcipotriol monotherapy (both used as needed) in terms of treatment adherence (71% vs 59%), PGA, and patient satisfaction. The rate of medication-related adverse events was also significantly lower with the combination.111
The use of salicylic acid in scalp psoriasis has been analyzed in various RCTs of moderate-low quality and short duration, mostly in free combination with corticosteroids but also as monotherapy, with good results.84,113,141,234–236
Although safety and efficacy analyses exist for other topical treatments, the volume and quality of published articles are low to moderate, and robust conclusions cannot be drawn.237
Psoriasis in folds and genitalsPsoriasis in folds significantly impacts the patients’ quality of life238 and presents treatment difficulties due to friction and moisture in these occluded areas, and because their skin is thinner and more sensitive.239 Therefore, and as stated in the technical data sheets, it is preferable to avoid higher potency corticosteroids.
- -
In these patients, the GPS recommends topical treatment with medium/low potency corticosteroids with close monitoring and maintenance strategies aimed at using the lowest possible doses (intermittent, on-demand treatments, etc.). Calcineurin inhibitors can be considered as an alternative.
A prospective observational study analyzed the efficacy of topical fluticasone 0.005% (medium potency corticosteroid) twice daily for 2 weeks in 20 patients with facial and flexural psoriasis.240 Tesults showed a 50% or greater improvement in the PGA (Physician Global Assessment). These results were maintained in most patients who continued treatment for another 8 weeks with once-daily application, 2 consecutive days per week. No adverse events of interest were described.240
A RCT analyzed 80 patients with flexural psoriasis treated for 4 weeks with topical betamethasone (high potency corticosteroid), calcipotriol, pimecrolimus 1%, or placebo once daily.92 The mean M-PASI (modified Psoriasis Area and Severity Index) score decreased by 86.4% in patients on betamethasone; by 62.4% in patients on calcipotriol; by 39.7% in patients on pimecrolimus; and by 21.1% in the placebo group. This improvement in M-PASI was significantly greater with betamethasone vs pimecrolimus, but there were no differences with calcipotriol, nor between calcipotriol and pimecrolimus. The mean VAS score for pruritus decreased by 78% in the betamethasone group; 57% with calcipotriol; 35% with pimecrolimus; and 43% with placebo. Finally, no significant safety problems were detected.92
Another RCT with intraindividual comparison in patients with different types of psoriasis, including flexural psoriasis, analyzed the application of 50μg/g calcipotriol vs 3μg/g calcitriol twice daily for 6 weeks.214 The response rate (total or almost total clearance) was significantly higher with calcitriol (67% vs 33%; p<0.05).214
A phase III RCT analyzed the efficacy profile of the combination calcipotriol 25μg/g with hydrocortisone (medium potency corticosteroid) vs tacalcitol 4μg/g ointment and placebo in 322 patients with flexural and facial psoriasis. The percentage of patients controlled at 8 weeks of treatment was 57% for the combination vs 46% for tacalcitol and 36% for placebo.241
Regarding calcineurin inhibitors, in a 6-week RCT of patients with flexural, genital, and facial psoriasis, TAS produced a greater percentage of clearance (according to PGA) vs calcitriol (60% vs 33%; p<0.05), with fewer local irritative adverse events.96 In another RCT of 167 patients with flexural and facial psoriasis, the frequency of “excellent” clinical improvement (defined as >90% in PGA) 8 weeks into therapy was significantly higher with TAS vs placebo (66.7% vs 36.8%; p=0.002). The TAS group also showed a higher frequency of lesion disappearance in folds than placebo (65.2% vs 31.5%; p<0.001).95 The results of an open-label study with topical TAS applied for 8 weeks in patients with facial and flexural psoriasis were very similar.224
In relation to pimecrolimus, an 8-week double-blind, placebo-controlled RCT with 57 patients with moderate-to-severe flexural psoriasis94 found that 71% of the pimecrolimus group achieved an IGA score of 0 or 1 vs 21% of the placebo group (p<0.001).94
Few data are available on the long-term efficacy and other treatments in these locations.219,222
Facial psoriasis- –
In patients with facial psoriasis, as in patients with flexural psoriasis, the GPS recommends topical therapy with medium/low potency corticosteroids with close monitoring and maintenance strategies aimed at using the lowest possible amounts (intermittent, on-demand treatments, etc.). Calcineurin inhibitors are recommended as an alternative and could also be used as corticosteroid-sparing agents in maintenance.
A 2-week regimen of topical fluticasone (medium potency corticosteroid) has shown significant clinical improvement according to PGA, maintained in most patients with subsequent intermittent treatment for another 8 weeks.240 Similar results have been reported with hydrocortisone.241
Regarding vitamin D analogs, in a 6-week RCT comparing calcitriol vs TAS, the percentage reduction in the mean target area score with calcitriol was 51% vs 67% with TAS (p<0.05). More patients achieved total or almost total clearance (according to PGA) with the calcineurin inhibitor (60% vs 33%; p<0.05), while local irritative events were also lower with TAS.96
In a double-blind, placebo-controlled RCT of 167 patients with flexural and facial psoriasis, “excellent” clinical improvement (defined as >90% in PGA) 8 weeks into therapy was significantly greater with TAS vs placebo (66.7% vs 36.8%; p=0.002),95 with similar results being reported in other studies.224,237
Long-term data and data on other topical therapies are scarce.69
Nail psoriasisNail psoriasis can also have a very significant impact on the patients’ quality of life, with alterations in fine motor skills or aesthetic, psychological, and social problems.242
- -
In nail psoriasis, the GPS recommends the use of very high/high potency corticosteroids and, as an alternative, calcineurin inhibitors or tazarotene. In individualized cases, occlusive dressings may also be considered.
In 2 double-blind, placebo-controlled RCTs, the efficacy profile of clobetasol 8% (very high potency corticosteroid) in colorless nail lacquer194 was analyzed, observing an improvement or complete resolution in 80% of patients. In this study, response was associated with the duration of treatment.194
The results of another RCT that included 15 patients with nail psoriasis showed the safety and efficacy profile of 3 concentrations of topical clobetasol in nail lacquer (0.05%, 1%, and 8%) twice daily for 16 weeks. The clinical response objectified with the Nail Psoriasis Severity Index (NAPSI) and mNAPSI was significantly greater with a higher concentration of clobetasol.243
A RCT compared the efficacy of topical clobetasol and tazarotene, both in cream and under nocturnal occlusion, for 12 weeks. Both drugs achieved a statistically significant improvement in pitting, onycholysis, hyperkeratosis, and salmon patches (relative to baseline), yet no inter-group differences were found.173
On the other hand, a small open-label study revealed that the fixed combination of calcipotriol/betamethasone dipropionate in ointment, once daily for 12 weeks, significantly improved NAPSI, hyperkeratosis, and nail onycholysis.244 The improvement in oil spots and pitting was moderate and mild, respectively.244
From a safety perspective, it should be noted that there are isolated reports of bone atrophy with chronic and persistent use of topical corticosteroids.244
Regarding vitamin D analogs, the results found in different RCTs with calcipotriol in patients with nail psoriasis have been modest.207,215,245
In relation to tazarotene, a RCT showed that its administration in gel for 24 weeks was significantly more effective than placebo in reducing onycholysis in occluded nails (weeks 4 and 12) and non-occluded nails (week 24),73 with a significant improvement in pitting in its occluded application (week 24), but without changes in non-occluded nails or other variables such as subungual hyperkeratosis, leukonychia, or nail growth rate.73 The combination of tazarotene with phototherapy has also proven effective in nail psoriasis.246,247 In contrast, no differences have been observed between tazarotene and clobetasol in this type of patients.173
In an open-label RCT of 21 patients with nail psoriasis, TAS 0.1% ointment was administered to some nails for 12 weeks, with the remaining nails serving as controls. The results showed significant differences in NAPSI in favor of TAS (mean absolute change of 13 points).248
Palmoplantar psoriasis- -
In palmoplantar psoriasis, the GPS recommends topical treatment with very high/high potency corticosteroids and, as an alternative, vitamin D analogs and occlusive dressings.
Palmoplantar psoriasis can be another difficult-to-treat location, for which there is little high-quality evidence.89,125,174,249,250
In an open-label RCT, 20 patients with palmoplantar psoriasis received mometasone ointment once daily until remission and for a maximum of 4 weeks; another 20 patients followed the same regimen but in combination with a new emollient.249 All patients showed improvement, with a significant reduction in severity (erythema, scaling, and infiltration) and affected surface area. Although the duration of corticosteroid therapy was similar between the groups, the improvement in scaling, affected surface area, and subjective symptoms was significantly greater in the new emollient group.249
Clobetasol in free combination with tar or phototherapy (PUVA) produced a significant PASI reduction in a RCT with patients with this type of psoriasis. In the palms, this PASI reduction was greater with the combination of corticosteroid and PUVA, and in the soles with corticosteroid and tar. The corticosteroid-tar combination did not cause relevant adverse events, while phototoxicity was observed in 22% of cases with topical PUVA.125
Another small RCT compared a 12-week regimen of topical therapy with clobetasol vs tazarotene, both in cream.250 Almost 53% of patients on tazarotene had complete skin clearance, as did 61.5% on topical clobetasol. There were no statistically significant differences between groups, nor significant problems regarding the safety of the treatments.250
A total of 29 patients with palmoplantar psoriasis were analyzed in a RCT that compared the safety and efficacy profile of occlusive dressing with calcipotriol ointment twice weekly vs non-occlusive treatment twice daily for 6 weeks.174 Both regimens were equally effective with improvement in the symptoms and signs of the disease, and without any relevant safety issues.174
Finally, in a RCT, 39 patients with palmoplantar psoriasis were randomized to receive an 8-week regimen of tar ointment vs salicylic acid ointment, which were left on overnight with gloves and socks.89 Improvement in both the symptoms and signs of palmoplantar psoriasis was observed in 75.5% of patients in the tar group vs 45.5% in the salicylic acid group (p<0.050). There were no adverse events of interest in either group.89
Pustular psoriasis- -
In patients with pustular psoriasis, the GPS recommends the use of very high/high potency topical corticosteroids, considering topical treatment as adjuvant, as in erythrodermic psoriasis.
Current evidence on the safety and efficacy profile of topical therapy in pustular psoriasis is scarce. A small crossover RCT demonstrated the efficacy profile of topical clobetasol under occlusion for 12 days.175 The results of another small RCT suggest that occlusive dressing with triamcinolone cream every 3 days could be effective.176
Erythrodermic psoriasis- -
Like pustular psoriasis, topical therapy in erythrodermic psoriasis is fundamentally adjuvant, and very high/high potency topical corticosteroids are the most recommended by the GPS.
Current evidence on the safety and efficacy profile of topical therapy in this type of patient is quite limited. A double-blind, placebo-controlled RCT showed that calcitriol petrolatum can improve lesions.68
Patient adherence and preferencesAdherence to topical treatments is crucial, as it is estimated that between 30% and 40% of applications are not performed, and between 8% and 88% of patients may be non-adherent, which significantly influences the efficacy of treatments.9–11 Lack of adherence is even greater in prolonged treatments, with 8 weeks being considered the critical point.9
Multiple SLRs have analyzed the factors associated with lack of adherence9–11 (Table 8), some non-modifiable, such as patient age or disease duration, but others susceptible to change (Table 9). On the other hand, the most important causes of lack of adherence, according to patients, are lack of efficacy, application time, and inadequate cosmetic characteristics of topical drugs (convenience).9
Factors associated with poor adherence to topical treatments in psoriasis.
| 1. Patient-related |
| Male gender |
| Earlier onset of psoriasis |
| Smoking, alcohol use |
| Lower socioeconomic status |
| Not being married |
| Being unemployed |
| Stress and a busy lifestyle |
| Fear of treatment side effects (especially corticosteroids) |
| Low trust in the physician |
| Frustration and treatment fatigue |
| 2. Topical treatment-related |
| Lack of efficacy |
| More than once-daily application |
| Combined treatments not in the same formulation |
| Complex regimens (e.g., one drug on weekdays, another on weekends) |
| Prolonged treatment duration |
| History of side effects from topical treatment |
| Not being the first topical treatment used by the patient |
| Type of vehicle (e.g., unpleasant texture, greasy, sticky, staining, odor) |
| Application difficulties |
| Time-consuming application |
| On-demand treatments |
| Interference with daily activities |
| 3. Psoriasis-related |
| Longer disease duration |
| Greater severity or disease impact |
| Facial psoriasis |
| Joint involvement |
| Involvement of non-visible areas |
| 4. Health care professional/system-related |
| Poor doctor–patient relationship |
| Not informing patients on treatment characteristics |
| Not teaching correct application technique |
| Drugs not covered by public health system or corresponding insurance |
Strategies to improve adherence to topical treatments in psoriasis.
| 1. Shared decision-making with the patient, considering preferences (vehicle selection, speed of action, etc.) |
| 2. Realistic expectation management (efficacy, time to effect, etc.) |
| 3. Patient education about treatment (goals, features, importance of following regimen) |
| 4. Selection of vehicle adapted to lesion location and patient characteristics/preferences |
| 5. Specific training on how to apply topical treatments |
| 6. Encouraging patient involvement in treatment |
| 7. Reassuring patients about topical treatment side effects (generally rare and mild, especially with proper use) |
| 8. Promoting the role of specialized nurses |
| 9. Close monitoring of patients suspected of poor adherence |
| 10. Use of educational materials in print and e-Health formats (mobile apps, videos, websites, etc.) |
In this regard, the GPS emphasizes the importance of selecting a topical treatment that not only adjusts to the severity/impact of psoriasis but also adapts to the characteristics and preferences of each patient (type of drug, regimen, and vehicle). The type of vehicle (base or excipient) can be decisive in adherence and satisfaction with topical treatment. Choosing a specific one can be fundamental for optimal adherence. The limited interest of professionals in the composition and nomenclature of topical galenic formulations can also negatively impact adherence.251
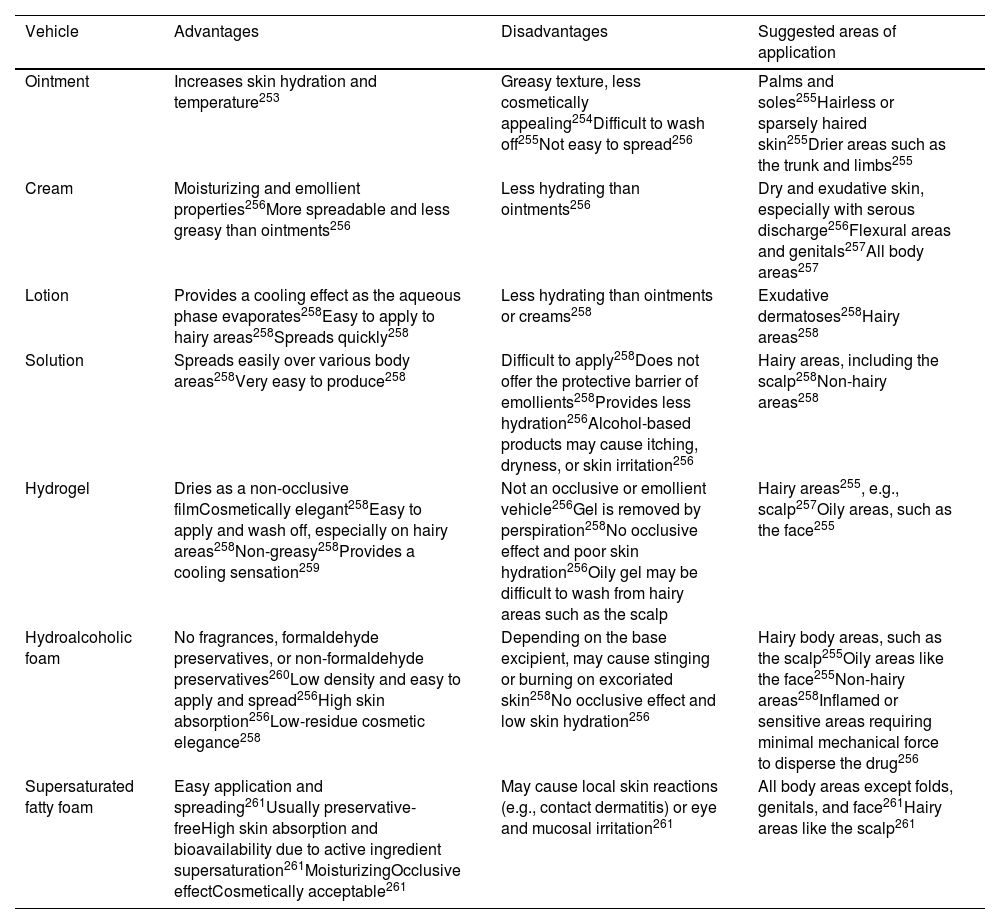
Table 10 provides an overview of the advantages, disadvantages, and suggested application areas for each galenic formulation of topical treatments.251,252 They are described in more detail in the supplementary data.
Overview of advantages, disadvantages, and suggested application areas of various topical formulations for psoriasis treatment.
| Vehicle | Advantages | Disadvantages | Suggested areas of application |
|---|---|---|---|
| Ointment | Increases skin hydration and temperature253 | Greasy texture, less cosmetically appealing254Difficult to wash off255Not easy to spread256 | Palms and soles255Hairless or sparsely haired skin255Drier areas such as the trunk and limbs255 |
| Cream | Moisturizing and emollient properties256More spreadable and less greasy than ointments256 | Less hydrating than ointments256 | Dry and exudative skin, especially with serous discharge256Flexural areas and genitals257All body areas257 |
| Lotion | Provides a cooling effect as the aqueous phase evaporates258Easy to apply to hairy areas258Spreads quickly258 | Less hydrating than ointments or creams258 | Exudative dermatoses258Hairy areas258 |
| Solution | Spreads easily over various body areas258Very easy to produce258 | Difficult to apply258Does not offer the protective barrier of emollients258Provides less hydration256Alcohol-based products may cause itching, dryness, or skin irritation256 | Hairy areas, including the scalp258Non-hairy areas258 |
| Hydrogel | Dries as a non-occlusive filmCosmetically elegant258Easy to apply and wash off, especially on hairy areas258Non-greasy258Provides a cooling sensation259 | Not an occlusive or emollient vehicle256Gel is removed by perspiration258No occlusive effect and poor skin hydration256Oily gel may be difficult to wash from hairy areas such as the scalp | Hairy areas255, e.g., scalp257Oily areas, such as the face255 |
| Hydroalcoholic foam | No fragrances, formaldehyde preservatives, or non-formaldehyde preservatives260Low density and easy to apply and spread256High skin absorption256Low-residue cosmetic elegance258 | Depending on the base excipient, may cause stinging or burning on excoriated skin258No occlusive effect and low skin hydration256 | Hairy body areas, such as the scalp255Oily areas like the face255Non-hairy areas258Inflamed or sensitive areas requiring minimal mechanical force to disperse the drug256 |
| Supersaturated fatty foam | Easy application and spreading261Usually preservative-freeHigh skin absorption and bioavailability due to active ingredient supersaturation261MoisturizingOcclusive effectCosmetically acceptable261 | May cause local skin reactions (e.g., contact dermatitis) or eye and mucosal irritation261 | All body areas except folds, genitals, and face261Hairy areas like the scalp261 |
Similarly, the GPS considers it essential to inform the patient about all aspects associated with treatment, from its objectives and characteristics (efficacy, speed of effect, risk management, etc.) to the importance of complying with the prescribed regimen and correctly performing the applications, to achieve greater therapeutic adherence.
Finally, the GPS emphasizes the need to periodically evaluate adherence to topical treatments through direct questions or specific questionnaires such as the Questionnaire for Adherence with Topical Treatments in Psoriasis (QATOP).262
The TTOP was designed to improve adherence and optimization of topical treatment in psoriasis and includes 5 actions159: (1) checklist for visits with instructions on face-to-face conversation between the dermatologist and the patient; (2) checklist for visits between nursing staff and the patient; (3) educational material for the patient; (4) telephone/e-mail support service for patients; and (5) treatment reminders, with contact from the nurse to the patient. In this study, the fixed combination of betamethasone dipropionate and calcipotriol in gel was used for up to 64 weeks. Compared to usual care, at 8 weeks, the response rate (total or almost total clearance according to PGA) was significantly higher in the TTOP group (36.3% vs 31.3%; p=0.026), differences that were maintained from week 8 to 64. In contrast, no differences were observed between groups in other variables such as BSA, patient assessment, or the DLQI.159 Patients evaluated the program with the Topical Therapy Adherence Questionnaire (TTAQ), observing a positive influence on various factors related to adherence.
ConclusionsTopical drugs are a fundamental element in the treatment of psoriatic patients. In recent years, advances have been made that require a re-evaluation of the role of these treatments, and although the published evidence on them is extraordinarily broad, its quality is highly variable. This also requires a reflection by experts to position this therapeutic modality in the most safe and effective way possible. In this document, we have generated a new framework to optimize the use of topical treatments based on the best available evidence and the experience of GPS experts.
FundingThis project was funded by the Spanish Academy of Dermatology and Venereology (AEDV).
CRediT authorship contribution statementMiquel Ribera, Esteban Dauden, Antonio Sahuquillo-Torralba, Lourdes Rodríguez-Fernández, Pablo De La Cueva, José Manuel Carrascosa were all involved in the conception and design of the study, the analysis and interpretation of the data, the drafting the article, and its critical review.
Conflicts of interestMiquel Ribera received consulting fees from Abbvie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gebro Pharma, Janssen, LEO Pharma, Eli Lilly, Novartis, Pierre-Fabre, SKB, UCB; payment or fees for lectures, presentations, manuscript drafting, or educational events from Abbvie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gebro Pharma, Janssen, LEO Pharma, Eli Lilly, Novartis, Pierre-Fabre, Pfizer, Sandoz, SKB, UCB; support to attend meetings and/or travel expenses from Abbvie, Almirall, Janssen, LEO Pharma, Eli Lilly, Novartis, and UCB.
Esteban Dauden received payment or fees for lectures, presentations, manuscript drafting, or educational events from Almirall, Janssen-Cilag, LEO Pharma, Novartis, Lilly, UCB, Boehringer-Ingelheim; support to attend meetings and/or travel expenses from UCB, Lilly, Janssen, LEO Pharma, and participated in advisory boards for Abbvie, Almirall, Janssen-Cilag, Novartis, Lilly, UCB, Brystol-Myers, and Boehringer-Ingelheim.
Antonio Sahuquillo-Torralba received grants or signed contracts with Novartis, UCB, Almirall, LEO Pharma, BMS, Janssen, Abbvie, Boehringer-Ingelheim; royalties or licenses from Novartis, UCB, Almirall, LEO Pharma, BMS, Janssen, Abbvie, Boehringer-Ingelheim; consulting fees from Novartis, UCB, Almirall, LEO Pharma, BMS, Janssen, Abbvie, Boehringer-Ingelheim; payment or fees for lectures, presentations, manuscript drafting, or educational events from Novartis, UCB, Almirall, Leo de Novartis, UCB, Almirall, LEO Pharma, BMS, Janssen, Abbvie, Boehringer-Ingelheim; support to attend meetings and/or travel expenses from Novartis, UCB, LEO Pharma, Janssen, Abbvie, and participated in advisory boards for Novartis, UCB, Almirall, LEO Pharma, BMS, Janssen, Abbvie, and Boehringer-Ingelheim.
Lourdes Rodríguez-Fernandez received consulting fees from Abbvie, Almirall, Amgen, Boehringer, Celgene, Janssen., LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, UCB, payment or fees for lectures, presentations, manuscript drafting, literary or educational events from Abbvie, Almirall, Amgen, Boehringer, Celgene, Janssen., LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, UCB; support to attend meetings and/or travel expenses from Abbvie, Almirall, Amgen, Boehringer, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, UCB, participated in advisory boards for Abbvie, Almirall, Amgen, Boehringer, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, and UCB.
Pablo de la Cueva received consulting fees from Abbvie, Almiral, Amgen, Boehringer, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, UCB, and payment or fees for lectures, presentations, manuscript drafting, or educational events from Abbvie Almiral, Amgen, Boehringer, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, and UCB.
Jose Manuel Carrascosa received grants or contracts from Janssen, Novartis, and Almirall, consulting fees from Abbvie, Novartis, Leo Farmacéutica, Sanofi, Janssen, UCB, BI, Lilly, Almirall, payment or fees for lectures, presentations, manuscript drafting, or educational events from Abbvie, Novartis, LEO Pharma, Sanofi, Janssen, UCB, BI, Lilly, and Almirall.