The electrosurgical unit is a very useful tool widely used in dermatology to treat benign and malignant skin lesions and to achieve hemostasis during surgery. However, precautions are required when this technique is used in patients with implantable electronic cardiac devices (IECD), such as pacemakers and defibrillators, because electromagnetic interference produced by the tool may cause such devices to malfunction. Before using electrosurgery in patients with IECDs, it is essential to ascertain the type of implanted device and the patient's level of dependence on it. The location of the skin lesion to be treated with respect to the device should also be assessed. Bipolar pacemakers are more resistant to interference. Appropriate monitoring and the use of bipolar forceps are recommended.
El electrobisturí es una herramienta muy útil y ampliamente utilizada en dermatología para el tratamiento de lesiones benignas y malignas cutáneas, y para la hemostasia durante la cirugía dermatológica. Su uso en pacientes con dispositivos electrónicos cardiacos implantables (marcapasos y desfibriladores) requiere tomar ciertas precauciones ya que puede producir interferencias electromagnéticas capaces de provocar su malfuncionamiento. Ante un paciente con uno de estos dispositivos se debe conocer el tipo de dispositivo que presenta, la dependencia del paciente, y valorar la localización tanto del dispositivo como de la lesión cutánea a tratar. El marcapasos en configuración bipolar es el más resistente a la interferencia. Se aconseja la monitorización adecuada del paciente y el uso de la pinza bipolar.
It is increasingly common in clinical practice to encounter patients with surgically treatable skin lesions who have a pacemaker or other implantable electronic cardiac device (IECD). The presence of such devices has classically been considered to contraindicate the use of the electrosurgical unit. However, owing to improved engineering, the resistance of these implantable devices to electromagnetic interference is constantly increasing. Some studies report a high level of safety for the use of the electrosurgical unit in dermatological surgery1,2 and, with certain exceptions, the fact that a patient is carrying an IECD should not substantially alter our surgery guidelines.
The goal of this article is to discuss the interference capacity of the different waves generated by an electrosurgical unit, and to explain the basic features of the different types of IECD, with a practical focus on highlighting the precautions that should be taken in this setting.
When dealing with a patient with an IECD, we need to know the answers to a number of questions (Table 1).
Questions That Must be Answered for Patients With Implantable Electronic Cardiac Devices.
| Does the patient wear a pacemaker or an AICD? |
| When was the device last monitored? |
| Is the patient dependent on the pacemaker? |
| Is the pacemaker single-chamber, dual-chamber, or triple-chamber? |
| Are the leads unipolar or bipolar? |
| In the case of an AICD, has the defibrillator function been activated recently? |
| Where is the lesion located? |
| When was the device implanted? |
Abbreviation: AICD, automatic implantable cardioverter-defibrillator.
We will begin by defining some important concepts and discussing the consequences of electromagnetic interference, and go on to discuss the preoperative, perioperative, and postoperative management of these patients.
DefinitionsElectrosurgeryElectrosurgery is a procedure that uses electrical energy to destroy tissue in an effective, fast, and economical manner, with immediately visible results. The procedure is performed using an electrosurgical unit, a device that transforms standard 60cycle current into a high-frequency current alternating at more than 200 000Hz.3–6 In dermatology, the procedure is used to achieve hemostasis during surgery and to treat and destroy benign and malignant lesions.
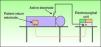
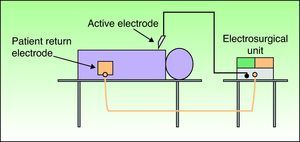
Types of CircuitThe circuit can be bipolar or monopolar. The most common practice is to use the electrosurgical unit with a monopolar circuit, in which case the patient forms part of the electrical circuit and the current passes through the patient's body (Fig. 1). In this configuration, the circuit comprises 4 elements: the generator, the active electrode, the patient, and the return electrode. The return electrode is extremely important because it completes the circuit, ensures patient safety, and prevents burns.3,6
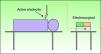
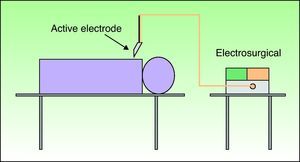
The use of bipolar electrosurgery is less common (Fig. 2). In the bipolar configuration, a special forceps is used and the current flows between the 2 tines, which serve as the active and return electrodes, allowing a complete circuit. The bipolar configuration is normally used in ophthalmology and neurosurgery and no patient return electrode is required.3–6
Types of WaveformThe electrosurgical unit can modify the waveform and voltage of the electrical current, thereby changing its effect on tissue and the speed at which heat is produced. The 3 standard waveforms used are a cutting current, a coagulation current, and a blended current. The power can also be adjusted.3–5
Interference CapacityAll electrical currents can interfere with the function of a pacemaker. The only technique that does not pose this problem is electrocautery, which, strictly speaking, is not a type of electrosurgery because no electric current passes through the patient's body. The instrument used in electrocautery is a filament heated to incandescence by means of a direct electric current; the technique can therefore be used safely in patients with pacemakers.2,7,8
The safest modality for electrosurgery is a bipolar circuit with a forceps because in this configuration the current does not flow through the patient's body and is therefore unlikely to interfere with the IECD.2,3,7–10
In the case of monopolar electrosurgery, there is some disagreement in the literature regarding the levels of interference associated with the different modes. In the opinion of some authors, the desiccation-fulguration mode is the least likely to interfere, followed by electrocoagulation and finally cutting.10,11 Other authors, however, consider the interference capacity of the cutting mode to be lower than that of the coagulation mode, as the voltage is lower.12
Implantable Electronic Cardiac DevicesThere are essentially 2 types of devices that we should be familiar with: pacemakers and automatic implantable cardioverter-defibrillators (AICD). Both detect intrinsic cardiac activity and respond according to a specific program.
PacemakersPacemakers are most often indicated in symptomatic bradycardia due to sinus node dysfunction or atrial ventricular block. They are identified by a code consisting of 3 to 5 letters(Table 2), in which each position relates to a specific type of information as follows: the first letter indicates the chamber paced, the second letter the chamber where the intrinsic rhythm is sensed, and the third the type of response to a sensed event. A fourth letter indicates programmability (whether the device has a rate modulation sensor), and the fifth indicates the chamber where multisite pacing is performed in implantable cardioverter-defibrillators.6,12,13
Pacemaker Codes.
| Letter 1Chamber paced | Letter 2Chamber sensed | Letter 3Type of response | Letter 4Rate modulation sensor | Letter 5Multisite pacing chamber |
| A=atrium | A=atrium | I=inhibited | R=rate adaptive | V=ventricle |
| V=ventricle | V=ventricle | T=triggered | O=none | A=atrium |
| D=dual (A + V) | D=dual (A + V)O=none | D=dual (I or T)O=none | D=dual (A+ V)O=none |
Pacemakers can be single-chamber or dual-chamber. Furthermore, they can be unipolar or bipolar.14 In bipolar devices the anode and cathode are located very close to each other on the distal end of the intracardiac lead. Pacemakers with bipolar leads, currently the most common type, have the advantage of being more resistant to electromagnetic interference.12
In unipolar devices, the anode is located at the tip of the intracardiac lead and the cathode is the casing of the pulse generator.
It is very important to determine whether the patient is pacemaker dependent (5%-10% of patients).7,10 Pacemaker dependency can be loosely defined as the inability to produce the intrinsic rhythm if stimulation is interrupted. Pacemaker dependent patients may experience problems if the delivery of impulses is inhibited or interrupted during surgery.6,7,12 Pacemaker dependency is normally indicated in the patient's cardiology report and on the European health care card of patients who have a pacemaker or AICD.
Automatic Implantable Cardioverter-DefibrillatorsAICDs are less common than pacemakers (only 1 in 500 devices) and are indicated in patients with a high risk of severe ventricular arrhythmia (ventricular tachycardia and ventricular fibrillation)10,12; they detect these arrhythmias and correct them by delivering bursts of high-energy electric shocks to defibrillate the heart. AICDs require particular care because malfunction could result in the delivery of inappropriate shocks with fatal consequences. In each case, it is essential to find out whether the device has been activated recently and when the last activation occurred.2,12
Consequences of InterferenceIn order to function correctly, modern pacemakers and AICDs have to detect intrinsic heart activity. Consequently, electrical signals from other sources, such as those generated by an electrosurgical unit, may be incorrectly interpreted by these devices as cardiac activity. This can provoke an inappropriate response, such as the inhibition of stimulus emissions (the problem most often observed). More rarely, with dual-chamber pacemakers, ventricular pacing may occur at a higher frequency than normal if the interference is interpreted as atrial activity. Asynchronous pacing can also occur (emission of stimuli at a fixed frequency regardless of cardiac activity) or response algorithms may be activated, leading the device to switch to automatic mode or ventricular safety pacing. Exceptionally, direct contact between the electrosurgical energy source and the pacemaker's pulse generator casing may damage the circuits or, if the current should pass through the pacemaker leads, cause heat injury to the heart and loss of stimulation.2,6,7,12,13,15
In AICDs, interference could provoke the inappropriate delivery of impulses or inhibition of the pacing system, which would leave the patient without protection in the event of asystole.9,12,15
Location of the LesionThe location of the skin lesion to be treated is a very important aspect that must be taken into account when considering electrosurgery in patients with IECDs. Use of an electrosurgical unit is not recommended in the immediate proximity of the device casing, that is, near the chest area.
When the electrosurgical unit is used in monopolar configuration, the electric current flows from the unit through the tip of the active electrode into the patient and returns to the unit via the patient return electrode. This return electrode should be placed close to the lesion and away from the cardiac device to ensure that the pathway followed by the current is short and directed away from the heart and the implanted device.3,6,10,12 For example, problems are unlikely to arise when the lesion is located below the umbilicus and the return electrode is placed under the patient's leg.9,12 In our patients, most of whom have lesions on the head and neck, we place the return electrode under the arm, shoulder or back.9 The choice of the right or left arm depends on which side the cardiac device is located. The tip of the active electrode should never be activated over the pacemaker and every effort should be made to keep it at least 15cm from the pulse generator and from the tip of the intracardiac pacemaker lead.7,14,15
Preoperative, Perioperative, and Postoperative PrecautionsThe patient should be assessed by the anesthesia and/or cardiology services. Before the operation, factors that might favor cardiac instability (anemia, abnormal electrolytes, etc.) should be investigated and any abnormalities found should be treated.2,6,11 The date on which the patient's device was last monitored should be ascertained. An electrocardiogram (ECG) and chest radiograph should be obtained. The ECG will provide indirect information regarding the patient's dependence and the configuration of the device (large spikes indicate a unipolar pacemaker).6,12 The chest radiograph will show whether the pacemaker is single-chamber or dual-chamber, the location of the leads, and whether they are unipolar or bipolar.6,10,12
RecommendationsPatients with IECDs undergoing electrosurgery must be monitored under the supervision of an anesthesiologist. The ECG should have at least 1 lead where the spike and/or QRS complex are fully visible and identifiable. The ECG should be complemented by a second monitoring method that is not affected by electrical interference (pulse oximetry).9,12
A bipolar configuration should be used when possible.1,3,6–9,12 The monopolar configuration should be used with moderation using the lowest power possible and brief, intermittent bursts to minimize the hemodynamic repercussions of any effect on the pacemaker.2,6,7,9,10,14
The locations of the lesions to be treated and their relation to that of the IECD should always be taken into account and the placement of the return electrode should be chosen to ensure that the current flows away from the heart and the device.3,7,10,12
When the unit is used to promote hemostasis, the current should not be activated until the electrode is in contact with the hemostat in order to prevent electric arcing.6,10,14
In pacemaker-dependent patients who have no intrinsic cardiac rhythm, if the pacemaker stops every time the electrosurgical unit is used a magnet can be applied that will cause most devices to switch to a fixed pace (asynchronous mode) and emit continuous pulses.2,9,11,14
In AICDs, a specialist in arrhythmias should be consulted and a request should be made to deprogram the device and reprogram it after the risk of electromagnetic interference has passed. The function that senses an arrhythmia and delivers corrective pulses should be deactivated and the pacemaker function should be maintained. The patient should be monitored and the adhesive paddles of an external defibrillator should be put in position so that an external defibrillator can be used if necessary.2,12
Ethical DisclosuresProtection of Human and Animal SubjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of DataThe authors state that no patient details appear in this article.
Right to Privacy and Informed ConsentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: García Bracamonte B, et al. Electrocirugía y dispositivos electrónicos cardiacos implantables (marcapasos y desfibriladores). Actas Dermosifiliogr. 2013;104:128-32.