Treatment of female pattern hair loss (FPHL) is challenging and conventional therapies are sometimes unsuccessful. Bicalutamide is a selective androgen receptor antagonist with higher affinity and a better safety profile than flutamide, often used in the management of prostate cancer.1,2 Recently, its use in the treatment of FPHL has grown, showing efficacy specially in premenopausal women with doses between 10 and 50mg daily.3–6 However, there is no scientific evidence regarding its therapeutic response and tolerability in postmenopausal women, a patient group with limited treatment options supported by high-quality scientific evidence.
We conducted a retrospective observational study to evaluate the therapeutic response and tolerance of oral bicalutamide in postmenopausal women. Forty postmenopausal women olde than 50 years with FPHL on a 6-month regimen of bicalutamide 30mg daily were included. The study was approved by the hospital Drug Research Ethics Committee (Sa-18579/19 – EC: 526). Trichoscan® was performed at baseline and after 6 months of treatment. Clinical standardized macroscopic images and 3 trichoscopic images at 8cm, 12cm and 18cm from the glabellar midpoint were obtained. Clinical response was evaluated by 2 independent dermatologists, using the Global Aesthetic Improvement Scale (GAIS: −2=greatly worsened, −1=worsened, 0=no change, +1=improved, +2=greatly improved). A survey on subjective response in hair density, hair loss and seborrhea and adverse effects was submitted to the patients.
The Q–Q plot and Shapiro–Wilk test were used to evaluate the distribution of the variables. Hair diameter and proportion of terminal and vellus hair had a normal distribution, therefore were assessed by Student's t. Olsen, Ludwig and Sinclair stages did not have normal distribution, therefore were assessed by Wilcoxon test.
The patients’ median age was 57 years old (range, 50–73). All patients were on oral antiandrogen therapy previously, all of them with dutasteride and 5% finasteride. All patients received concomitant treatment with oral minoxidil (mean dose, 2.1mg daily) and 95% mesotherapy with dutasteride or bicalutamide and/or platelet-rich plasma injections once a month. They had been receiving concomitant therapies for at least 1 year prior, and the antiandrogen was switched to bicalutamide due to lack of efficacy. No other changes were made to their treatment regimen.
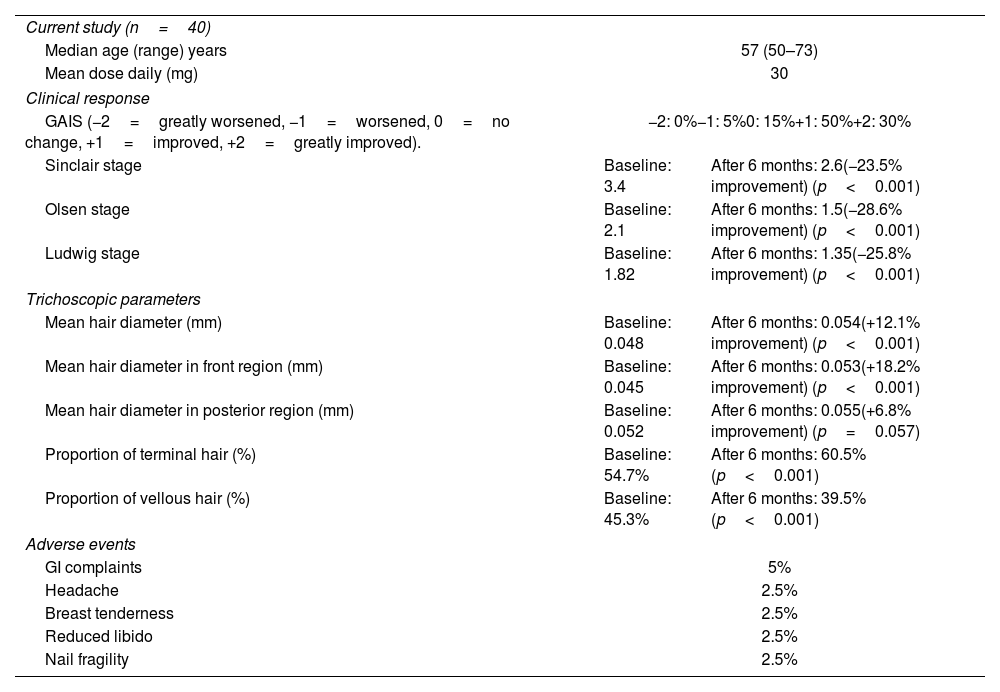
Patient demographics, clinical and trichoscopic response and adverse events are shown in Table 1. Mean result of GAIS in macroscopic response was +1.1 points. A total of 30% of the patients showed significant improvement, 50% improved, 15% showed no change, and 5% worsened. The mean Olsen stage at baseline and after 6 months of treatment was 2.1 and 1.5, respectively. The mean Ludwig stage at baseline and after 6 months was 1.82 and 1.35, respectively. The mean Sinclair stage at baseline and after 6 months was 3.4 and 2.6, respectively. The improvements in Olsen, Ludwig, and Sinclair stages correspond to 28.6%, 25.8%, and 23.5% increases in hair density, respectively (Fig. 1).
Patient demographics, clinical and trichoscopic response and adverse events.
| Current study (n=40) | ||
| Median age (range) years | 57 (50–73) | |
| Mean dose daily (mg) | 30 | |
| Clinical response | ||
| GAIS (−2=greatly worsened, −1=worsened, 0=no change, +1=improved, +2=greatly improved). | −2: 0%−1: 5%0: 15%+1: 50%+2: 30% | |
| Sinclair stage | Baseline: 3.4 | After 6 months: 2.6(−23.5% improvement) (p<0.001) |
| Olsen stage | Baseline: 2.1 | After 6 months: 1.5(−28.6% improvement) (p<0.001) |
| Ludwig stage | Baseline: 1.82 | After 6 months: 1.35(−25.8% improvement) (p<0.001) |
| Trichoscopic parameters | ||
| Mean hair diameter (mm) | Baseline: 0.048 | After 6 months: 0.054(+12.1% improvement) (p<0.001) |
| Mean hair diameter in front region (mm) | Baseline: 0.045 | After 6 months: 0.053(+18.2% improvement) (p<0.001) |
| Mean hair diameter in posterior region (mm) | Baseline: 0.052 | After 6 months: 0.055(+6.8% improvement) (p=0.057) |
| Proportion of terminal hair (%) | Baseline: 54.7% | After 6 months: 60.5% (p<0.001) |
| Proportion of vellous hair (%) | Baseline: 45.3% | After 6 months: 39.5% (p<0.001) |
| Adverse events | ||
| GI complaints | 5% | |
| Headache | 2.5% | |
| Breast tenderness | 2.5% | |
| Reduced libido | 2.5% | |
| Nail fragility | 2.5% | |
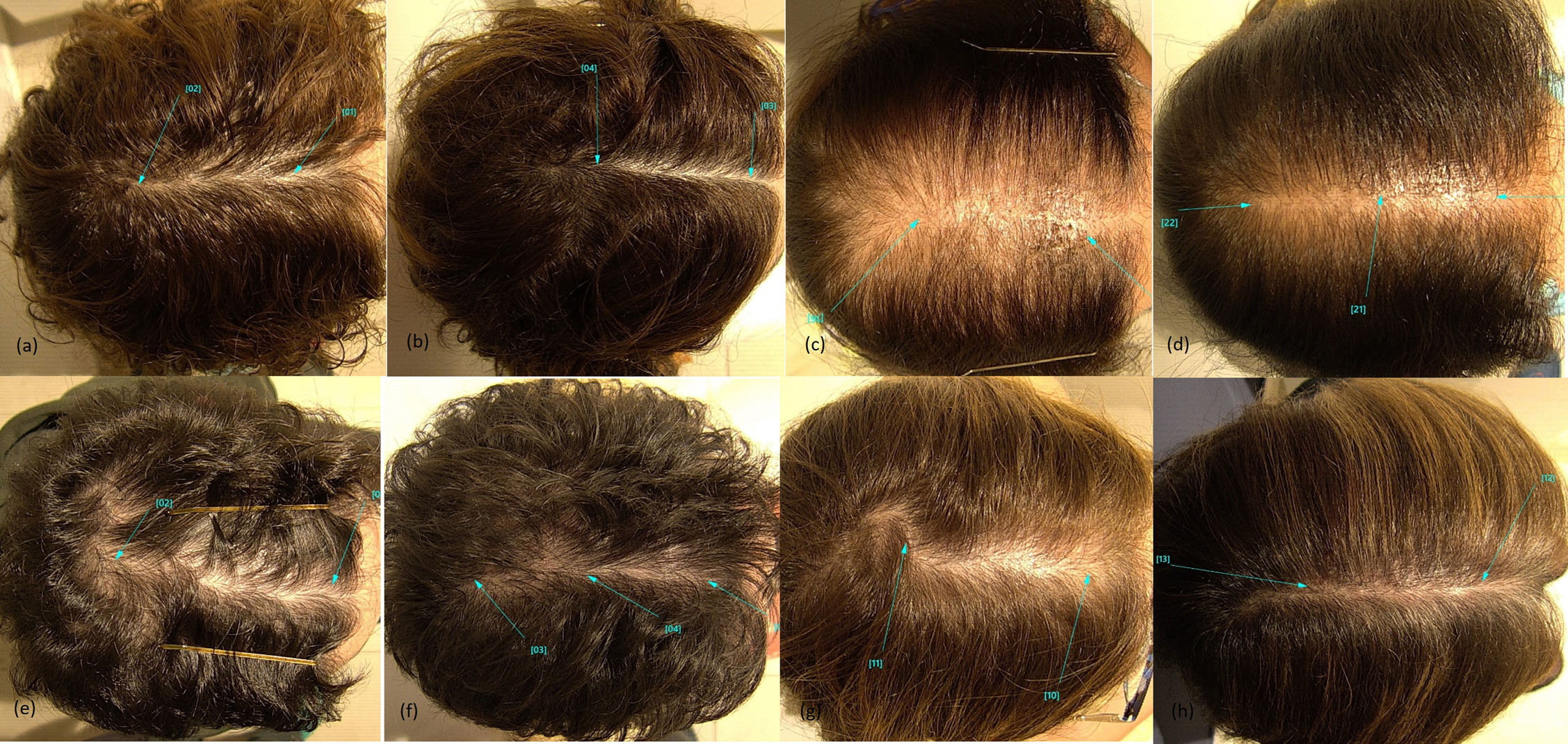
Case #1: (a) FPHL in postmenopausal woman at baseline. (b) Clinical improvement 6 months into bicalutamide. Case #2: (c) FPHL in postmenopausal woman at baseline. (d) Clinical improvement 6 months into bicalutamide. Case #3: (e) FPHL in postmenopausal woman at baseline. (f) Clinical improvement 6 months into bicalutamide. Case #4: (g) FPHL in postmenopausal woman at baseline. (h) Clinical improvement 6 months into bicalutamide.
Regarding trichoscopic parameters, there was an increase of 12.1% in the mean hair diameter, from 0.048mm at baseline to 0.054mm after 6 months. There was a greater improvement in the front region of scalp (18.2% increase; from 0.045mm to 0.053mm) compared to posterior region (6.8% increase; from 0.052mm to 0.055mm). Proportion of terminal hair increased from 54.7% at baseline to 60.5%, parallel with the decrease of vellus hair, from 45.3% at baseline to 39.5%.
Global satisfaction of the patients, in a scale of 1 to 10, was 7.1. Satisfaction in seborrhea improvement, hair density and hair loss was 7, 6.5 and 6.4 respectively. Mean number of hair washes per week reduced from 3.9 to 2.6, proving the usefulness of bicalutamide treating seborrhea.
The rate of adverse effects was 12.5%, all of them mild without the need for dose reduction or interruption of treatment. Reported adverse effects were abdominal discomfort or swelling (5%), headache (2.5%), breast tenderness (2.5%), decreased libido (2.5%) and nail fragility (2.5%).
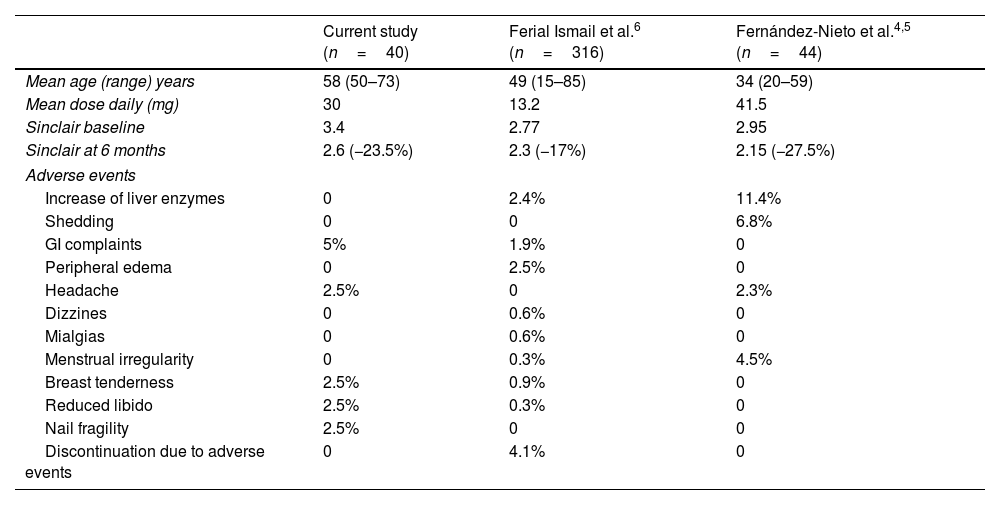
Results of our study are consistent with those of former studies,4–6 although some differences were found. Comparison of current evidence in the literature is shown in Table 2. One of the key points is the mean age of the patients. We hypothesize that the 30mg dose has a favorable balance between good therapeutic response and good tolerance. Bicalutamide was discontinued in three patients (7.5%) due to lack of response in our study.
| Current study (n=40) | Ferial Ismail et al.6 (n=316) | Fernández-Nieto et al.4,5 (n=44) | |
|---|---|---|---|
| Mean age (range) years | 58 (50–73) | 49 (15–85) | 34 (20–59) |
| Mean dose daily (mg) | 30 | 13.2 | 41.5 |
| Sinclair baseline | 3.4 | 2.77 | 2.95 |
| Sinclair at 6 months | 2.6 (−23.5%) | 2.3 (−17%) | 2.15 (−27.5%) |
| Adverse events | |||
| Increase of liver enzymes | 0 | 2.4% | 11.4% |
| Shedding | 0 | 0 | 6.8% |
| GI complaints | 5% | 1.9% | 0 |
| Peripheral edema | 0 | 2.5% | 0 |
| Headache | 2.5% | 0 | 2.3% |
| Dizzines | 0 | 0.6% | 0 |
| Mialgias | 0 | 0.6% | 0 |
| Menstrual irregularity | 0 | 0.3% | 4.5% |
| Breast tenderness | 2.5% | 0.9% | 0 |
| Reduced libido | 2.5% | 0.3% | 0 |
| Nail fragility | 2.5% | 0 | 0 |
| Discontinuation due to adverse events | 0 | 4.1% | 0 |
Although bicalutamide has been used in other conditions such as polycystic ovary syndrome and severe hirsutism, showing mild and well-tolerated adverse effects at the 18-month follow-up,2 it is necessary to maintain a close monitoring to detect potential long-term adverse effects.
Limitations of our analysis are its sample size, retrospective design and use of concomitant therapies.
In conclusion, oral bicalutamide might be a promising therapeutic option with a good tolerance for postmenopausal women with FPHL. No studies have ever been found regarding the therapeutic response and tolerance of oral bicalutamide in postmenopausal women. Therefore, further research in this field is needed.
Conflict of interestThe authors declare that they have no conflict of interest.