Direct-acting oral anticoagulants (DOACs) have emerged as safer, easier-to-manage alternatives to traditional vitamin K antagonists and are used increasingly because they require no monitoring, have a wider therapeutic window, and react less with other drugs. However, there is little consensus on optimal perioperative management when these drugs are used in dermatologic surgery. This article describes the characteristics of DOACs and reviews current evidence on their use in this setting.
Los anticoagulantes orales directos (ACOD) emergen como una alternativa más cómoda y segura que los clásicos antagonistas de la vitamina K (AVK); no precisan monitorización, poseen una ventana terapéutica más amplia y tienen menos interacciones farmacológicas. Sin embargo, a pesar de que su uso está cada vez más extendido, existe poco consenso sobre cuál es su manejo perioperatorio óptimo en cirugía dermatológica. En este artículo se describen las características de los ACOD y se revisa la evidencia disponible sobre su uso perioperatorio en la cirugía de la piel.
Approximately 1.3% of the population in Spain are estimated to be taking anticoagulants. Perioperative management of patients treated with traditional coumarin anticoagulants, that is, vitamin K antagonists (VKAs), is well known. However, an increasing number of patients who undergo surgical procedures are taking new oral anticoagulants such as direct-acting oral anticoagulants (DAOCs) and perioperative management in these situations is less standardized.1,2 These agents are emerging as a more convenient and safer alternative to VKAs, given that they do not require routine monitoring, the therapeutic window is wider, the anticoagulant effect is more predictable, there are fewer drug-drug interactions, and the risk of intracranial bleeding is lower, all with a similar efficacy compared with traditional anticoagulants.3,4 Approved indications include the prevention and treatment of thromboembolic complications of nonvalvular atrial fibrillation, prophylaxis and treatment of deep vein thrombosis and pulmonary embolism, and, in the case of rivaroxaban, atherothrombotic prevention after acute coronary syndrome.5–7
The limited clinical experience of the use of these agents in patients undergoing cutaneous surgery and the publication of differing opinions have led to uneven perioperative management among dermatologists.8 Some authors suggest that the intervention should be delayed until treatment is complete while others propose management analogous to VKAs.9 However, the pharmacological profile, monitoring, risk of bleeding, and mechanism of action of these agents differ substantially from VKAs.
A questionnaire distributed to dermatologists in the United Kingdom on the management of DOACs found that the points of least consensus were actions prior to complex surgical interventions and the time for which the drug should be suspended.10
Given that their use is increasingly widespread, it is essential to know the characteristics of these agents, as well as the means available to reverse their effects.
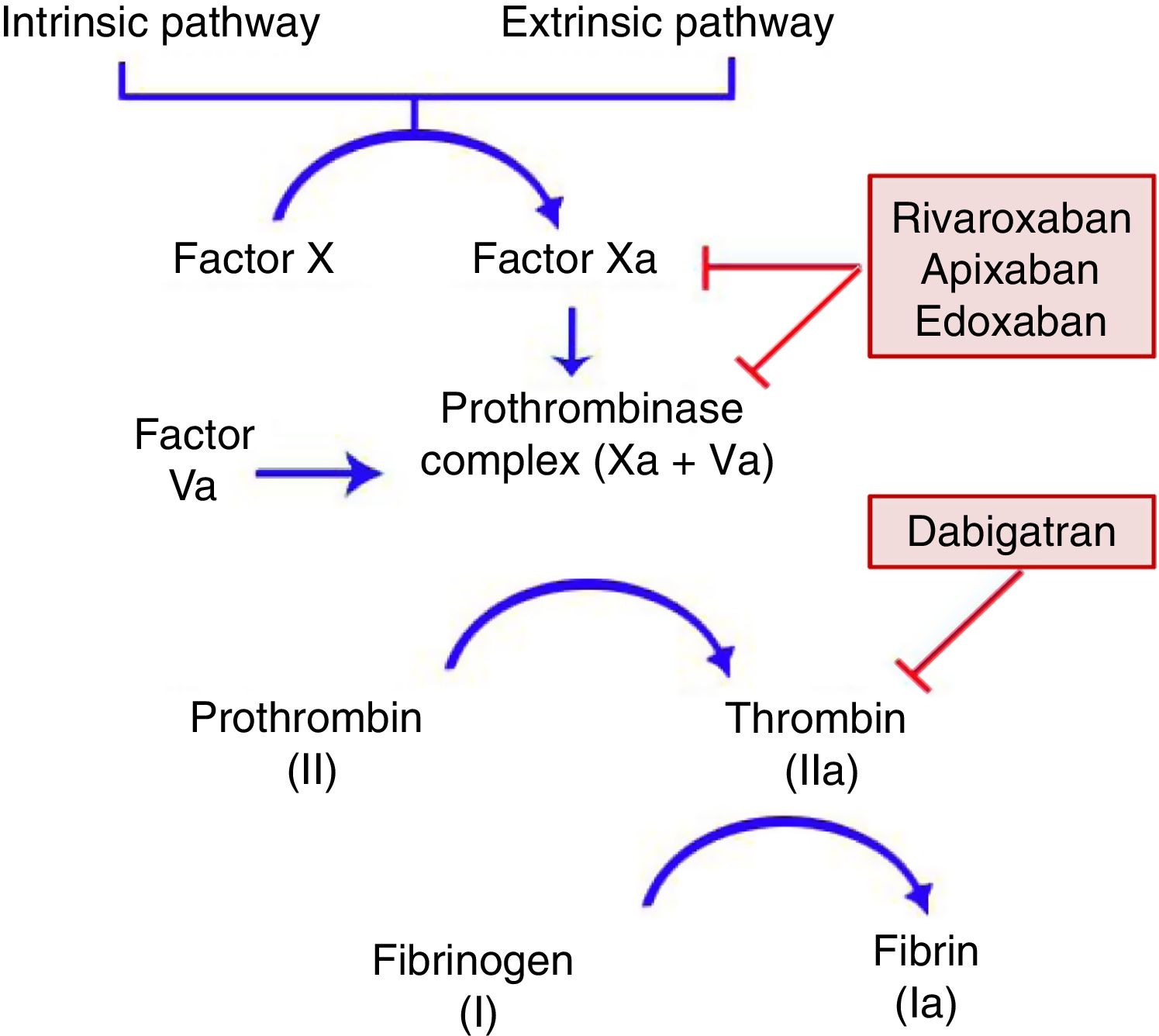
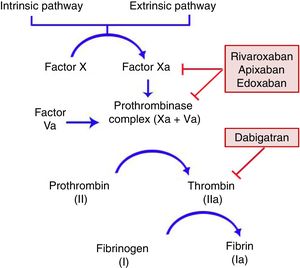
General CharacteristicsMechanism of ActionDOACs act on a point in the common coagulation pathway, after the intrinsic and extrinsic paths meet on activation of factor X, which binds to activated factor V to form the prothrombinase complex. This in turn gives rise to activated factor II or thrombin. Finally, factor IIa is responsible for activating fibrinogen (Fig. 1).11,12 Dabigatran (Pradaxa®) is the only direct and reversible oral inhibitor of thrombin, whereas rivaroxaban (Xarelto®), apixaban (Eliquis®), and edoxaban (Lixiana®) are inhibitors of activated factor X (Xa), both in free form and bound to prothrombinase.
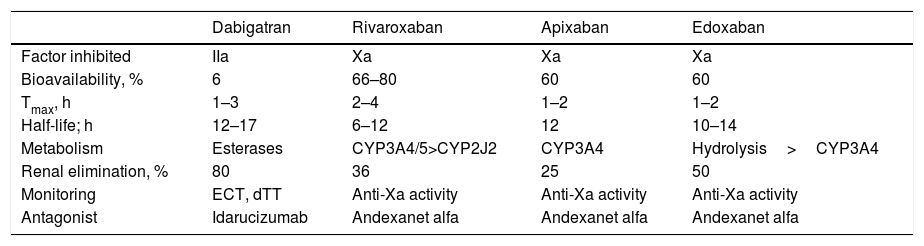
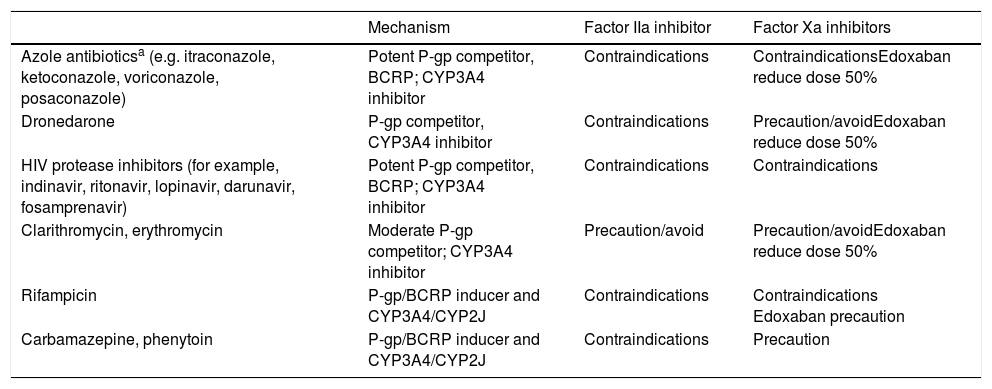
The DOACs act more quickly than VKAs and their effects are less long-lasting. The peak of maximum activity occurs 2–4h after administration and they have a half-life of between 8 and 18h in patients with normal renal function (Table 1).4,11,13 The main drug-drug interactions of DOACs are with inhibitors and inducers of the P-glycoprotein (P-gp) transport system or the CYP3A4 system (Table 2).6,14
Pharmacokinetic and Antagonist Properties of DOACs.
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|
| Factor inhibited | IIa | Xa | Xa | Xa |
| Bioavailability, % | 6 | 66–80 | 60 | 60 |
| Tmax, h | 1–3 | 2–4 | 1–2 | 1–2 |
| Half-life; h | 12–17 | 6–12 | 12 | 10–14 |
| Metabolism | Esterases | CYP3A4/5>CYP2J2 | CYP3A4 | Hydrolysis>CYP3A4 |
| Renal elimination, % | 80 | 36 | 25 | 50 |
| Monitoring | ECT, dTT | Anti-Xa activity | Anti-Xa activity | Anti-Xa activity |
| Antagonist | Idarucizumab | Andexanet alfa | Andexanet alfa | Andexanet alfa |
Abbreviations: CYP, cytochrome P450; DOACs, direct oral anticoagulants; dTT, plasma diluted thrombin time; ECT, ecarin clotting time; Tmax, time to maximum plasma concentration; IIa, activated factor II; Xa, activated factor X.
Main Drug-Drug Interactions of DOACs.
| Mechanism | Factor IIa inhibitor | Factor Xa inhibitors | |
|---|---|---|---|
| Azole antibioticsa (e.g. itraconazole, ketoconazole, voriconazole, posaconazole) | Potent P-gp competitor, BCRP; CYP3A4 inhibitor | Contraindications | ContraindicationsEdoxaban reduce dose 50% |
| Dronedarone | P-gp competitor, CYP3A4 inhibitor | Contraindications | Precaution/avoidEdoxaban reduce dose 50% |
| HIV protease inhibitors (for example, indinavir, ritonavir, lopinavir, darunavir, fosamprenavir) | Potent P-gp competitor, BCRP; CYP3A4 inhibitor | Contraindications | Contraindications |
| Clarithromycin, erythromycin | Moderate P-gp competitor; CYP3A4 inhibitor | Precaution/avoid | Precaution/avoidEdoxaban reduce dose 50% |
| Rifampicin | P-gp/BCRP inducer and CYP3A4/CYP2J | Contraindications | Contraindications Edoxaban precaution |
| Carbamazepine, phenytoin | P-gp/BCRP inducer and CYP3A4/CYP2J | Contraindications | Precaution |
Metabolism-inducing drugs increase thromboembolic risk, whereas inhibitors/competitors increase the risk of bleeding
Abbreviations: BCRP, breast cancer resistance protein; CYP, cytochrome P450; DOACs, direct oral anticoagulants; P-gp, P glycoprotein; IIa, activated factor II; Xa, activated factor X.
DOACs do not require routine monitoring for coagulation because the pharmacokinetics and pharmacodynamics are predictable and there are few drug-drug interactions. Furthermore, assays for precise monitoring of these variables are costly and are not available in all clinics (Table 1). They are therefore reserved for invasive interventions and emergency ones; such interventions are the exception within the field of dermatology.15,16
Prothrombin time and international normalized ratio (INR), used for monitoring VKAs, do not correlate linearly with the anticoagulation effect of DOACs.17
Assessment of Perioperative Risk of BleedingThe perioperative risk of bleeding is determined by the patient's comorbidities and by the characteristics of the surgical procedure.
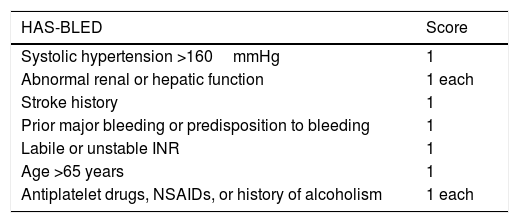
For appropriate assessment of the risks associated with the patient's comorbidities, scales are available such as the HAS-BLED (Table 3), which can be used by dermatologic surgeons to satisfactorily characterize risk.18 One example is that the presence of high perioperative systolic blood pressure is associated with a greater rate of intraoperative bleeding in Mohs surgery.19
HAS-BLED Score.
| HAS-BLED | Score |
|---|---|
| Systolic hypertension >160mmHg | 1 |
| Abnormal renal or hepatic function | 1 each |
| Stroke history | 1 |
| Prior major bleeding or predisposition to bleeding | 1 |
| Labile or unstable INR | 1 |
| Age >65 years | 1 |
| Antiplatelet drugs, NSAIDs, or history of alcoholism | 1 each |
A score of 3 or more points is highly predictive of presenting a bleeding process.
HAS-BLED, hypertension, abnormal liver/renal function, stroke history, bleeding history or predisposition, labile INR, elderly, drug/alcohol usage.
Abbreviations: INR, international normalized ratio; NSAID, nonsteroidal antiinflammatory drug.
Source: Adapted from Doherty et al.18
The risk of relevant bleeding, that is, bleeding that is not self-limiting or cannot be controlled using a compress, is low in dermatologic surgery.20–23
If the risk is analyzed specifically for patients with DOACs, there is an increase in relevant bleeding after major surgical inventions, but no increase in minor ones.24–27 Some studies have analyzed risk of bleeding by anatomical site, regardless of the extent of the procedure, with varying and somewhat inconsistent results. Thus, currently, anatomical site is not considered a determining factor.21,28 In the case of Mohs surgery, this technique has not been shown to be associated with a greater risk of bleeding per se.29
The dermatologic surgeon should be aware of and assess the factors associated with a significant increase in risk of bleeding (Table 4), paying particular attention to those procedures that carry a greater risk of onset of subcutaneous hematomas, which can dissect the tissue to form a virtual cavity. Examples of such interventions are selective sentinel lymph node biopsy, interventions that require closure with large flaps such as complete cheek advancement flap, and interventions in the oral cavity.30
Cutaneous Surgical Procedures Associated with Greater Postoperative Risk of Bleeding.
| Interventions that involve multiple layers (fascia, muscle, cartilage, bone) |
| Large surgical defect (>4cm) or closure with large graft/flap |
| Interventions with limited visual access to the surgical field (liposuction, lymph node, anorectal, or oral cavity interventions) |
Source: Adapted from Sporbeck et al.30
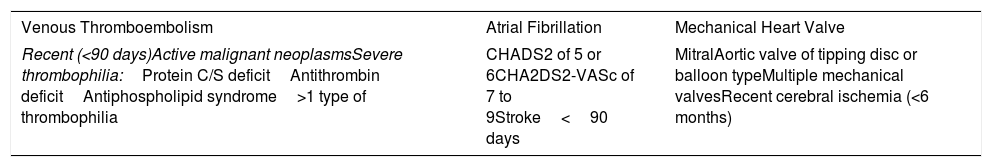
There are factors that are associated with an increased risk of presenting with a thromboembolic event after suspension of the anticoagulant. The presence of some of these characteristics is responsible for a thromboembolic risk of at least 10% in absence of anticoagulation (Table 5).31–33
Clinical Situations With High Risk of Bleeding.
| Venous Thromboembolism | Atrial Fibrillation | Mechanical Heart Valve |
|---|---|---|
| Recent (<90 days)Active malignant neoplasmsSevere thrombophilia:Protein C/S deficitAntithrombin deficitAntiphospholipid syndrome>1 type of thrombophilia | CHADS2 of 5 or 6CHA2DS2-VASc of 7 to 9Stroke<90 days | MitralAortic valve of tipping disc or balloon typeMultiple mechanical valvesRecent cerebral ischemia (<6 months) |
CHA2DS2: congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischemic attack (TIA); VASc: vascular disease, age 65 to 74 years, sex category.
Among the thromboembolic complications secondary to perioperative suspension of anticoagulation in dermatologic surgery, there have been reports of cerebrovascular accidents, myocardial infarction, pulmonary embolism, deep vein thrombosis, and retinal artery occlusion with secondary blindness.34
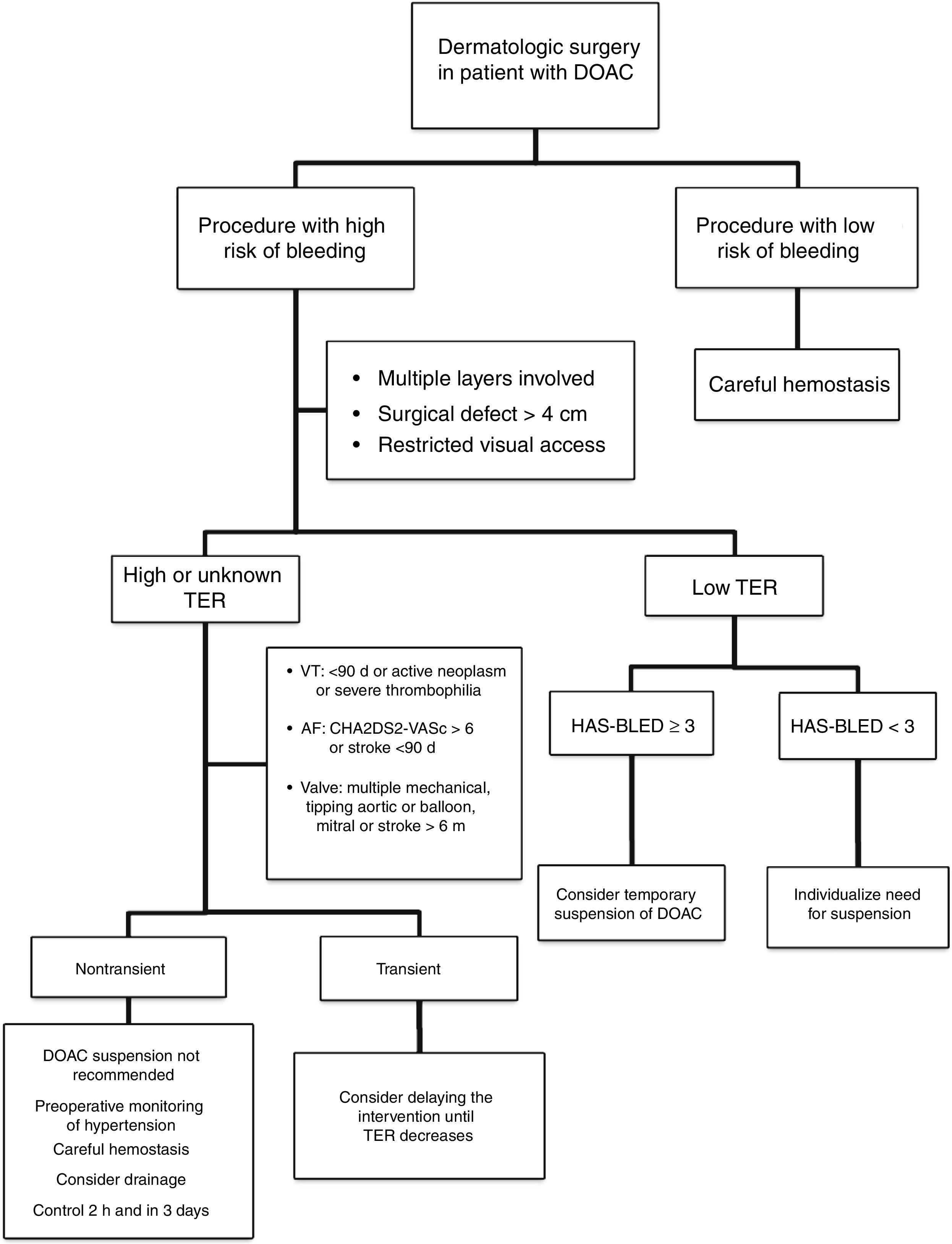
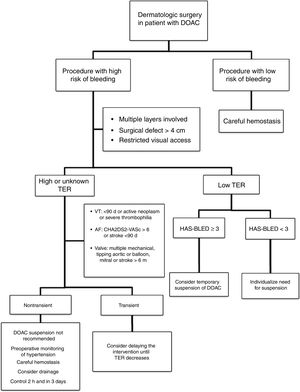
Approach in Dermatologic SurgeryThe evidence supporting perioperative management of DOACs is still limited, given that the results of randomized clinical trials or large observational studies are still not available. Currently, it is accepted that anticoagulation should not be suspended prior to procedures that are associated with a low risk of bleeding. In clinical practice, some surgeons support a delay in taking DOACs until after the intervention.35 If the surgical risk of bleeding is considered high (given the type of intervention and/or comorbidities of the patient), and the thromboembolic risk is low; the most accepted strategy would be to temporarily suspend the DOAC (Fig. 2).18,32 If we do not know the thromboembolic risk, or if this is high, anticoagulation should not be routinely suspended and, ideally, consensus should be reached with the medical team on the approach to hemostasis and coagulation.32,33 It should be remembered that the threat to life inherent in a thromboembolic complication and its management exceed the threat of perioperative bleeding in a dermatologic procedure, as this is usually readily treated.36,37 One option in those patients who have a transient high thromboembolic risk (for example, stroke or venous thromboembolism in the past 90 days) would be to delay the intervention until the risk has returned to normal.
Careful monitoring of hemostasis forms part of basic management in all surgical interventions. Surgery should be performed carefully, trying to spare tissue as far as possible, with use of bipolar or monopolar electric scalpels, or other electrocauterizing devices to ensure adequate hemostasis in small vessels. For larger vessels (>2mm), electrocoagulation may be insufficient, and a different technique used, with ligature preferred over suture given its longer duration of action. If diffuse bleeding occurs at the edge of the incision, the horizontal mattress stitching and continuous locking stitching have been shown to have a greater hemostatic effect.38
Additional MeasuresAdditional hemostatic measures should be taken in patients who have not suspended DOAC therapy. Systematic use of nonischemic compression bandages are recommended in these patients and, in large interventions, placement of drains should be considered. Preoperative optimization of blood pressure contributes to a decrease in the risk of intraoperative bleeding. It is advisable to perform an initial examination of the wound 2h after the intervention if anesthetics have been used with vasoconstrictors and, in complex interventions, the wound should be reassessed after 3 days. Furthermore, the patient should be duly informed of the potential risk of bleeding and the measures that should be taken should bleeding occur.38,39
Perioperative SuspensionThere is evidence to support that the risk of bleeding is associated with the trough drug concentration and not the peak concentration.40
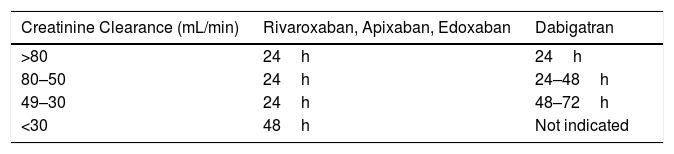
Thus, before the intervention, it is recommended to suspend the drug for a period of at least 2 half-lives of elimination to leave a residual effect varying between 12% and 25%.35 In patients with impaired renal function, creatinine clearance should be calculated and the suspension time should be adjusted (Table 6) according to the ACOD used (level of evidence 2D).15,18
If hemostasis has been effective, the DOAC can be reintroduced between 6 and 12h after the procedure (level of evidence 2C).15,18,41
Given that these drugs have a short half-life and a rapid onset of action, perioperative bridging therapy is not required. In fact, some studies suggest that this is not recommended given the increase in postoperative bleeding with no decrease in thromboembolic risk.42,43
Postoperative HemostasisThe majority of bleeding complications after dermatologic surgery can be controlled with local measures given the ready access to the surgical wound. Measures include local cooling, elevation of the operated region, changing of compression bandages, use of topical hemostatic agents, and, in cases of persistent or excessive bleeding, surgical examination of the wound and use of electrocoagulation.44
Depending on the severity of bleeding and failure of the aforementioned measures, other general support measures can be applied, such as saline administration, transfusion of blood products, and temporary suspension of the DOAC.13 In view of the short half-life and early plasma peak, the effect of these agents declines quickly after the first hour.
Only exceptionally and in situations of excessive, uncontrolled, life-threatening bleeding would there be a need for other measures that reduce the plasma levels of the DOAC or reverse its effect.
These measures include the use of activated charcoal, provided it is taken between 1 and 6 hours after DOAC administration. Nonspecific reversal agents (complexes of prothrombin concentrates that contain up to 4 activated coagulation factors) and tranexamic acid (subcutaneously or endovenously administered antifibrinolytic) can also be used. Before administration of such agents, a history of thromboembolic events, a family history of thrombophilia, or the concomitant use of hormonal contraceptives should be ruled out. Hemodialysis has proved effective for reversing the effects of dabigatran.45–49
Finally, there are specific antidotes for DOACs that are in different phases of development. Only idarucizumab (Praxbind®), a noncompetitive inhibitor of dabigatran, is approved by the European Medicines Agency to reverse its effects in emergency surgical interventions with life-threatening bleeding complications.50
ConclusionsThe limited evidence available suggests that there is some increased risk of bleeding in patients in therapy with DOACs who undergo dermatologic surgical interventions. However, suspension could increase the thromboembolic risk and should be a consensus decision taken on an individual basis. The ideal candidate for suspending a DOAC is the patient with a low thromboembolic risk but with a high tendency for bleeding who will undergo surgery that is associated with a high risk of bleeding. The duration of this suspension will depend on the drug and renal function, and particular attention should be paid to possible pharmacological interactions. In contrast, procedures with low risk of bleeding can be performed without suspending anticoagulation. Nevertheless, it is recommended to apply strict hemostatic measures with compression bandages. In the case of large surgical defects, strict postoperative controls should be performed. When a bleeding complication occurs that is not controlled with local measures, suspension of the DOAC should be considered. In the case of life-threatening bleeding, an exceptional situation in dermatologic surgery, idarucizmab, a specific approved antidote, can be employed to reverse the effect of dabigatran.
Please cite this article as: Cabezas-Calderon V, Bassas Freixas P, García-Patos Briones V. Anticoagulantes orales directos en cirugía dermatológica. Actas Dermosifiliogr. 2020;111:357–363.