Dermoscopy is a noninvasive technique that has been demonstrated to improve diagnostic accuracy in basal cell carcinoma (BCC). The first dermoscopic model for the diagnosis of BCC, based mainly on the identification of pigmented structures, was described by Menzies and colleagues, and since then dermoscopy has generated an abundance of literature useful to routine clinical practice. From a practical perspective, dermoscopic structures associated with BCC can be classified as pigmented, vascular, or nonpigmented/nonvascular. One of the most recent applications of dermoscopy in BCC is as an aid to predicting histologic subtype and essentially differentiating between superficial and nonsuperficial BCC. It can also, however, help raise suspicion of more aggressive variants with a higher risk of recurrence. A thorough dermoscopic examination during follow-up of patients with actinic damage or a history of multiple BCCs can facilitate the detection of very incipient lesions and significantly impact treatment and prognosis.
La dermatoscopia es una técnica no invasiva que ha demostrado mejorar la precisión diagnóstica en el carcinoma basocelular (CBC). Desde la descripción inicial llevada a cabo por Menzies y colaboradores, basada fundamentalmente en hallazgos asociados a la presencia de pigmento, se ha generado una importante cantidad de bibliografía útil en la práctica clínica diaria. De una forma práctica, podemos clasificar las estructuras dermatoscópicas asociadas al diagnóstico de CBC en estructuras pigmentadas, vasculares y no pigmentadas/no vasculares. Una de las aplicaciones más recientes de la dermatoscopia en el CBC trata de pronosticar el subtipo histológico mediante el examen dermatoscópico y permite fundamentalmente diferenciar entre el CBC superficial y no superficial. Sin embargo, la dermatoscopia también nos permite sospechar variantes agresivas con mayor riesgo de recurrencia. La exploración dermatoscópica exhaustiva en el seguimiento de pacientes con daño actínico o historia de múltiples CBC posibilita la detección de lesiones muy incipientes, con repercusiones terapéuticas y pronósticas importantes.
Basal cell carcinoma (BCC) is the most common malignant tumor in humans; its incidence is rising and it is associated with significant morbidity and costs.1 The estimated incidence of BCC in Spain is approximately 250 cases per 100 000 person-years.2,3
Dermoscopy is a noninvasive technique that has been demonstrated to improve diagnostic accuracy in BCC. It has become an essential tool in dermatologic practice. Dermoscopic examination improves diagnostic accuracy by helping to differentiate BCCs from other tumors and inflammatory diseases and by providing a reliable means of distinguishing between different histologic subtypes of BCC.4,5 Early diagnosis may have a key role in reducing disease- and treatment-related morbidity.6 In addition, the costs of primary treatment have been found to increase with tumor size.7 Adequate training in dermoscopy is necessary for the early diagnosis of BCC.4
Dermoscopy in BCCThe first dermoscopy findings in BCC, described by Menzies and colleagues, were largely based on the identification of pigmented structures. According to this model, BCC can only be diagnosed in the absence of a pigment network and in the presence of at least 1 of the following structures: blue-gray ovoid nests, maple leaf–like areas, a spoke-wheel pattern, ulceration (not associated with recent trauma), and arborizing telangiectasias. The model showed a sensitivity of 97% for the diagnosis of pigmented BCC and a specificity of 93% and 92% for differentiating BCC from invasive melanoma and benign pigmented skin lesions, respetively.8
Subsequent studies confirmed the reproducibility and reliability of this early model.6 Since then, however, an abundance of literature has emerged, giving rise to new dermoscopic criteria and evidence that have contributed to further improving the dermoscopic diagnosis of BCC.
On a practical level, dermoscopic structures associated with the diagnosis of BCC can be classified into 1) pigmented structures, 2) vascular structures, or 3) nonpigmented, nonvascular structures9 (Table 1).
Main Dermoscopic Structures in Basal Cell Carcinoma.a
| Pigmented Structures | Vascular Structures | Other Structures |
|---|---|---|
| Blue-gray ovoid nests | Arborizing telangiectasias | Multiple erosions |
| Blue-gray globules | Short fine telangiectasias | Ulceration |
| Blue-gray dots | Shiny white-red areas | |
| Maple leaf–like structures | Shiny white structures | |
| Spoke-wheel structures | ||
| Concentric structures |
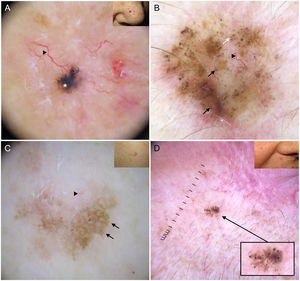
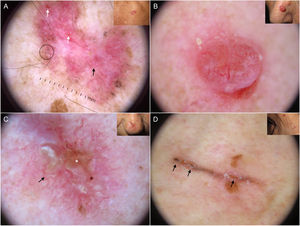
Blue-gray ovoid nests, which are confluent ovoid or elongated structures, are the largest pigmented structures observed in BCC10 (Fig. 1A). Histologically, they correspond to large tumor nests with pigment aggregates invading the dermis.11 Blue-gray globules are similar but smaller and appear as dispersed round or oval structures10 (Fig. 1B). Their histologic correlates are small tumor nests in the papillary and/or reticular dermis.11 Blue-gray dots are seen as randomly distributed dots in sharp focus (Fig. 1B). They correspond to small tumor aggregates at the dermoepidermal junction or in the superficial dermis, although they may also reflect free pigment deposits or melanophages at the junction.10,11
Dermoscopic structures in basal cell carcinoma (BCC). A, Infiltrative BCC; note the large blue-gray ovoid nest (white asterisk) and arborizing telangiectasias (triangle). B, Pigmented infiltrative BCC with multiple blue-gray globules (black arrows), blue-gray dots (white arrow), and arborizing telangiectasias (triangle). C, Pigmented superficial BCC with fine telangiectasias (triangle) and maple leaf–like areas (black arrows). D, Pigmented 2-mm BCC with multiple concentric structures.
Maple leaf–like areas are bulbous extensions connected to a common base at the periphery of the tumor. They never arise from a pigment network and are highly specific to BCC9,10 (Fig. 1C). Spoke-wheel structures, which are also highly specific to BCC, consist of radial projections connected to a more strongly pigmented central axis.10 These projections are occasionally poorly defined and appear as globular structures with a darker center; in such cases, they are called concentric structures (Fig. 1D). Maple leaf–like areas, spoke-wheel structures, and concentric structures all correspond to pigmented nests at the dermoepidermal junction and in the superficial papillary dermis.11
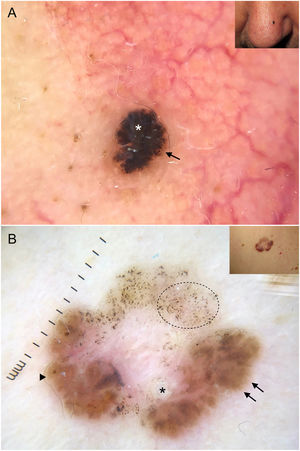
It is important to note that strongly pigmented BCCs may show dermoscopic features associated with melanocytic lesions, such as brown globules, a blue-white veil, peppering (multiple gray dots), or, less frequently, a pigment network or projections6 (Fig. 2A, B). Reflectance confocal microscopy can have an important role in detecting BCCs with clinical and dermoscopic features reminiscent of melanoma.12
Basal cell carcinomas with features of melanocytic lesions. A, Strongly pigmented 3-mm lesion with a blue-white veil (white asterisk) and brown globules (black arrow). B, Multicomponent pattern in a basal cell carcinoma in regression: multiple gray dots (circle with dotted line), projections (black arrows), maple leaf–like areas (triangle), and milia-like cyst (asterisk); shiny white structures and polymorphous vascularization.
Arborizing telangiectasias are the main vascular structure in BCC and have a positive predictive value of 94%.13 They are bright red vessels, usually no longer than 1 mm, that branch irregularly into finer capillaries. They are sharply focused as they are located on the surface of the tumor10 (Fig. 1A, B). Histologically, they correspond to tumor neovascularization in the form of dilated vessels in the dermis.
Short fine telangiectasias are the second most common vascular structure in BCC. These vessels do not exceed 1 mm in length, have very few branches, and correspond to telangiectatic dermal vessels9,10 (Fig. 1C).
BCCs may occasionally show other vascular structures that are typically seen in other types of tumors, including melanocytic lesions. Examples are dotted vessels, irregular linear vessels, and hairpin or comma vessels.6
Other StructuresThe observation of orange-red structureless areas in BCC indicates the presence of ulceration; these areas correspond to a total loss of the epidermis and a partial loss of the superficial dermis.9 Erosions, in turn, are seen as reddish-brown or yellow-orange areas, smaller than those associated with ulceration. Histologically, they correspond to focal necrosis of the epidermis.14
Shiny white-red structureless areas correspond to diffuse dermal fibrosis or fibrotic tumor stroma.9,10
Shiny white structures can only be visualized by polarized light. They may appear as short orthogonal white lines (shiny white streaks or chrysalis). In BCC, however, they tend to be disorganized or arranged in parallel. The combination of shiny white lines or streaks and shiny white areas is highly suggestive of nonpigmented BCC; short white orthogonal lines, by contrast, are more indicative of melanoma.15,16Although their histological correlation is unclear, shiny white-red structures appear to correspond to dermal fibrosis or fibrotic tumor stroma.11
Recently Described Dermoscopic CriteriaA number of potentially useful dermoscopic criteria for clinical practice have been recently published.
One example is a circumferential stellate pattern extending from the visible margin of the tumor; it may be formed by white lines, vessels, or skin surface folds. This pattern has been linked to infiltrative BCC.17
Negative maple leaf–like structures are a subtle dermoscopic sign that can help delineate tumors, essentially in nonpigmented BCC. They are nonpigmented bulbous structures, morphologically comparable to maple leaf–like areas, that interrupt structures in the surrounding skin (e.g., telangiectasias and solar lentigines).18
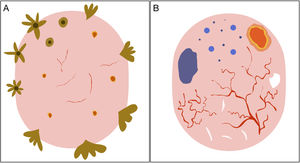
Dermoscopic Structures by Histologic SubtypeDermoscopy is an excellent tool for creating a nexus between clinicians and pathologists as most dermoscopic features have direct histologic correlates.11 A number of authors have recently investigated the extent to which dermoscopy is capable of discriminating between the different histologic subtypes of BCC19,20 (Figs. 3 and 4). This distinction, primarily between low-risk BCC and aggressive subtypes, has important treatment implications.21,22
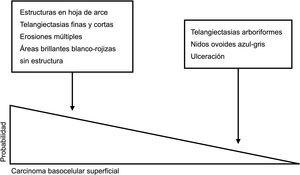
Dermoscopic clues for distinguishing between superficial and nonsuperficial basal cell carcinoma. Maple leaf–like structures, short fine telangiectasias, multiple erosions, and shiny white-red structureless areas are powerful predictors of superficial basal cell carcinoma. Arborizing telangiectasias, blue-gray ovoid nests, and ulceration by contrast, are predictors of other histologic subtypes.5,9
Schematic representation of dermoscopic features of superficial and nonsuperficial basal cell carcinoma. A, Superficial basal cell carcinoma: maple leaf–like structures, spoke-wheel structures, concentric structures, multiple erosions, and short fine telangiectasias. B, Nonsuperficial basal cell carcinoma: arborizing telangiectasias, blue-gray ovoid nest, multiple blue-gray dots and globules, ulceration, and whitish structures.
Low-risk BCCs generally consist of superficial and nodular BCCs. These tumors typically have an indolent behavior and are associated with a lower risk of recurrence than more aggressive subtypes.22
Superficial BCCAccording to the dermoscopic model proposed by Lallas et al.,5 the combined presence of maple leaf–like areas and short thin telangiectasias together with the absence of blue-gray ovoid nests, arborizing telangiectasias, and ulceration, is highly predictive of superficial BCC. An algorithm developed by the authors to discriminate between this variant and other histologic subtypes showed a sensitivity of 81.9% and a specificity of 81.8%.5
According to a recent systematic review, the most common dermoscopic structures in superficial BCC are short fine telangiectasias (60%), multiple erosions (43%), and shiny white structures (43%) against a white-red structureless background (79%).20 Blue-gray dots and globules are the most common finding in pigmented lesions (27%); maple leaf–like areas are observed in 25% of cases. Spoke-wheel and concentric structures, while quite specific for superficial BCC, are less common.5,20,23
Fine telangiectasias, spoke-wheel structures, and erosions have been independently linked to superficial BCC located on the trunk24 (Fig. 5A).
Subtypes of basal cell carcinoma (BCC). A, Superficial BCC: spoke-wheel areas (circle), fine telangiectasias (black arrow), and multiple erosions (white arrows); note the shiny white-red structureless areas and white structures. B, Nodular BCC: arborizing telangiectasias and whitish structures. C, Morpheaform BCC with scant branching (black arrow) located mainly at the edge of the tumor and associated with central ulceration (white asterisk). D, Linear BCC with multiple erosions (black arrows) following a skin fold in the context of cutis rhomboidalis.
Arborizing telangiectasias are the main dermoscopic finding in nodular BCC. They are present in 75% of cases; the next most common features are shiny white structures (43%) and ulceration (31%)20 (Fig. 5B). Dermoscopic examination of pigmented nodular BCC typically shows blue-gray ovoid nests (36%), usually in combination with arborizing telangiectasias.5,20 Less common pigmented structures are blue-gray globules and dots.10
High-Risk Histologic SubtypesHigh-risk histologic subtypes have ill-defined borders, an invasive growth pattern, and a tendency towards perivascular and perineural invasion. Complete excision thus is more difficult, meaning recurrence rates are higher.22 Generally speaking, aggressive BCCs appear to have a smaller pink area and a relative absence of vessels in the central part of the tumor.25
Morpheaform BCCMorpheaform (sclerodermiform) BCC may show dermoscopic structures at a later stage of development than other subtypes.26 Seventy-five percent of tumors show a structureless hypopigmented (porcelain) area. Arborizing telangiectasias are also common in morpheaform BCC (51%); they tend to have less evident branching and to be finer and more dispersed20,27 (Fig. 5C). Morpheaform BCCs are rarely pigmented, but when they are, dermoscopy generally shows blue-gray ovoid nests.20
Infiltrative BCCInfiltrative BCC usually shows arborizing telangiectasias (76%) and ulceration (44%).20 Other common findings are shiny red areas, multiple blue-gray globules, and fine telangiectasias22,23 (Fig. 1A, B). Although multiple erosions are mainly observed in superficial BCC, a significant association has also been detected in infiltrative BCC.22
Micronodular Basal Cell CarcinomaFew studies have specifically analyzed the dermoscopic features of micronodular BCC, but there have been reports of truncated vessels and multiple blue-gray globules.28,29
Basosquamous CarcinomaIn a series of 22 basosquamous carcinomas, Giacomel et al.30 found that the most common dermoscopic features were unfocused peripheral arborizing vessels (73%), keratin masses (73%), superficial scales (68%), ulceration or blood crusts (68%), white structures (64%), and blue-gray blotches (59%). All but 1 of the lesions had at least one dermoscopic feature of BCC and another of squamous cell carcinoma.
The authors of a subsequent retrospective study of 36 basosquamous carcinomas found that keratin masses, present in 91.7% of tumors, were the most common dermoscopic structure. Other common features were surface scaling (77.8%), ulceration (69.4%), white structureless areas (69.4%), white clods (66.7%), and blood spots on keratin masses (66.7%). A polymorphous vascular pattern was detected in 61% of lesions, the vast majority of which had arborizing telangiectasias. The authors also reported rosettes in 11.1% of lesions and concluded that basosquamous carcinoma is characterized by a vascular pattern suggestive of BCC and dermoscopic features of keratinization.31
Other SubtypesFibroepithelioma of PinkusFibroepithelioma of Pinkus is characterized by fine arborizing telangiectasias with less evident branching; dotted vessels and white streaks may also be observed. Pigmented variants show brown-gray structureless areas and blue-gray dots. Other common findings are milia-like cysts and ulceration.32 A more recently described pattern is the white network, also known a negative pigment network.33
Linear BCCLinear BCC is a rare morphologic variant of BCC sometimes seen in association with different histologic subtypes. A number of factors could explain its linear appearance, including limited lateral growth due to the presence of dermal fibrosis, interactions between the stroma and Langer lines, and the Koebner phenomenon. Linear BCC may show any of the dermoscopic features associated with BCC in general34 (Fig. 5D).
Dermoscopy in the Management of BCCDermoscopic erosions and ulceration have been shown to be predictive of response to imiquimod in BCC, with investigations showing a 7-fold higher probability of complete response in the presence of a single erosion, a 38-higher probability in the presence of multiple erosions, and an 8-fold higher probability in the presence of ulceration.35
Dermoscopy can also reveal pigmented structures in 30% of clinically nonpigmented BCCs, providing thus additional information that could influence the use of certain treatments such as photodynamic therapy.36
Dermoscopic examination after nonablative treatment in BCC has also proven useful for the evaluation of residual tumor. The presence of residual dermoscopic features (pigmented structures, ulceration, and arborizing telangiectasias) is a reliable indicator of tumor persistence.37,38 White-red structureless areas and fine surface telangiectasias can be monitored over time, and the absence of dermoscopic features of superficial BCC is strongly predictive of complete histologic clearance.38
Early Dermoscopic Diagnosis of BCCBCC grows slowly, about 0.5 mm every 10 weeks. This slow growth, coupled with the tumor’s reduced metastatic potential, generally portends a good prognosis. Diagnostic delays, however, can often influence treatment, treatment-associated costs, and prognosis. The current evidence supports the value of early diagnosis, especially in the H-zone of the face, as even a small increase in size can have significant therapeutic and prognostic repercussions.39 The surgical excision of small lesions, for instance, is relatively simple, but larger lesions may require complex surgery associated with high morbidity and may even be inoperable.
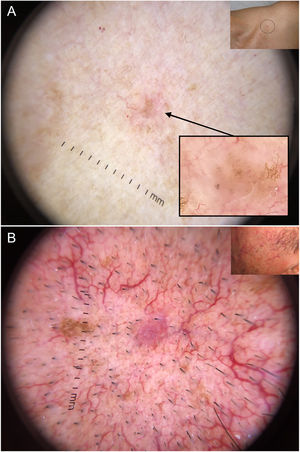
Dermoscopy has proven to be a reliable tool for detecting small BCCs (< 5 mm), as this tumor, unlike melanocytic lesions, typically shows dermoscopic features from the time of onset40,41 (Fig. 6). In a study by Longo et al.,40 only ulceration and multiple erosions were significantly more common in larger BCCs (> 5 mm). Finally, pigmented structures such as blue-gray ovoid nests and multiple blue-gray dots can play a crucial role in the early detection of lesions that would otherwise be very difficult to detect.40–42
Very small basal cell carcinomas (BCCs). A, 2-mm micronodular BCC, detected by the presence of pigmented structures (multiple blue-gray dots); note the interruption of the surrounding skin structures (telangiectasias and lentigines). B, 3-mm nodular BCC with fine telangiectasias and hairpin vessels.
Dermoscopy is an essential tool in the differential diagnosis of BCC. Its usefulness goes beyond the detection of more or less evident lesions as it provides a reliable means of distinguishing between different histologic subtypes and can also detect very incipient lesions. Early diagnosis of BCC in patients with actinic damage results in simpler surgery and lower morbidity and treatment-associated costs.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: M. Álvarez-Salafranca, M. Ara, P. Zaballos, Dermatoscopia del carcinoma basocelular: revisión actualizada. Actas Dermosifiliogr. 2021;112:330–338.